Article Text
Abstract
Objectives To compare the incidence and drivers of major adverse kidney events (MAKEs) between COVID-19 and non-COVID-19 acute respiratory distress syndrome (ARDS) patients, with a focus on long-term kidney outcomes.
Design Retrospective cohort study.
Setting Single-centre intensive care unit in the Midlands, UK.
Participants 708 ARDS patients (458 COVID-19, 250 non-COVID-19).
Primary and secondary outcome measures The primary outcome was MAKE at 365 days (MAKE-365), defined as new renal replacement therapy (RRT), estimated glomerular filtration rate (eGFR) <75% of baseline or all-cause mortality. Secondary analyses examined non-mortality MAKE components.
Results The incidence of MAKE-365 was significantly higher in the non-COVID-19 group compared with the COVID-19 group (66% vs 39%, p<0.001), primarily driven by increased RRT initiation, followed by mortality and eGFR decline (p=0.055). Independent predictors of MAKE-365 included lower eGFR and elevated bilirubin in both groups. Age (p<0.001) and diabetes (p=0.041) were additional predictors in the COVID-19 cohort, while lower albumin (p=0.002) was significant in the non-COVID-19 group. Excluding mortality, RRT and eGFR decline remained significant drivers of MAKE outcomes in the non-COVID-19 cohort.
Conclusions Non-COVID-19 ARDS patients face a greater risk of MAKE-365 and adverse kidney outcomes due to higher RRT requirements and mortality rates. These findings underscore the importance of tailored interventions and long-term nephrology follow-up, particularly for patients with reduced eGFR, elevated bilirubin and comorbidities like diabetes and hypoalbuminaemia.
- Intensive Care Units
- Respiratory Distress Syndrome
- Acute renal failure
- COVID-19
- INTENSIVE & CRITICAL CARE
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request. The de-identified participant datasets analysed for this study are available on reasonable request from the corresponding author at d.parekh@bham.ac.uk.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Intensive Care Units
- Respiratory Distress Syndrome
- Acute renal failure
- COVID-19
- INTENSIVE & CRITICAL CARE
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study addresses long-term kidney outcomes in acute respiratory distress syndrome, providing a direct comparison of COVID-19 and non-COVID-19 cohorts.
MAKE at 365 days was used as the primary outcome, aligning with internationally recognised nephrology definitions.
The large sample size, consistent management and lengthy follow-up strengthen the reliability of findings.
The single-centre design may limit generalisability, and differences in intensive care unit admission criteria could introduce selection bias.
Baseline estimated glomerular filtration rate estimates and a lack of competing risk analysis may impact the precision of results.
Background
The clinical course of COVID-19 ranges from mild, transient infections to severe conditions leading to multi-organ failure and mortality, particularly in vulnerable individuals.1 2 Acute kidney injury (AKI) has been notably prevalent among patients with COVID-19 who develop acute respiratory distress syndrome (ARDS), affecting up to 59% of these patients, with approximately 20% requiring renal replacement therapy (RRT). The mortality associated with AKI in these patients is significantly higher than those without AKI.3
Major adverse kidney events (MAKEs), comprising the need for RRT, a reduction in estimated glomerular filtration rate (eGFR) of less than 75% of baseline, or all-cause mortality, are crucial for assessing the long-term impacts of AKI. These measures are relevant for understanding the medium to long-term outcomes in ARDS patients.4 Furthermore, the assessment of MAKE at 365 days (MAKE-365) provides a critical lens for evaluating 1-year kidney outcomes, offering valuable insights into the prolonged effects of severe illness.
Previous studies have shown that patients with AKI or renal dysfunction at intensive care unit (ICU) admission have a high risk for progression to receive RRT, which is one of the components of the MAKE outcome.4 Several studies have investigated the long-term kidney outcomes in patients who developed AKI during COVID-19 hospitalisation. For instance, a study by Lu et al5 found that 58.0% of hospitalised COVID-19 patients with AKI experienced early recovery, 14.8% delayed recovery and 27.1% prolonged recovery.5 Longer time to recovery from AKI was associated with higher incidences of major adverse cardiovascular events, MAKE, recurrent AKI and new-onset chronic kidney disease (CKD) within 90 days postdischarge. This highlights the persistent risk of adverse kidney outcomes long after the acute phase of the infection has resolved.
Similarly, Nugent et al compared the rate of change in eGFR after hospital discharge between patients with and without COVID-19 who experienced in-hospital AKI.6 They found that patients with COVID-19-associated AKI had a greater decrease in eGFR over time compared with non-COVID-19 AKI patients, indicating a worse long-term renal prognosis.
Most recently, a large multicentre study by Aklilu et al7 provided additional insights into the long-term outcomes of COVID-19-AKI survivors.7 Their findings revealed that COVID-19-AKI survivors had a 33% lower risk of MAKE, including worsened kidney function and mortality, compared with patients with AKI from other illnesses. Contrary to initial concerns, the study suggested that COVID-19-AKI survivors might experience more favourable long-term kidney outcomes compared with non-COVID-19-related AKI.
Despite the prevalence of AKI in ARDS during COVID-19, there is a notable gap in research comparing long-term kidney outcomes between ICU ARDS admissions with and without COVID-19. This study specifically aims to identify the incidence of MAKE-365 from ICU admission in these two patient groups, which is vital for understanding and potentially mitigating long-term renal consequences in this high-risk cohort.
Methods
Study design and participants
This retrospective cohort study included ICU patients with ARDS, both COVID-19 and non-COVID-19, at Queen Elizabeth Hospital Birmingham. Ethical approvals for non-COVID-19 ARDS patients were obtained from the Wales Research and Ethics Committee 1 (REC 16/WA/0169) under the Alveolar Macrophage in ARDS (AM-ARDS) study, with subsequent approval by the Health Research Authority and University Hospitals Birmingham and Heart of England NHS Trusts (National Institute for Health and Care Research (NIHR) Portfolio, registration number 32296). Local governance approval was granted by the audit and quality improvement departments for the collection of observational data from patients with COVID-19.
In brief, this retrospective study included adult patients (aged ≥18 years) admitted to ICU and receiving invasive mechanical ventilation (IMV) who met ARDS diagnostic criteria based on the Berlin Definition.8 The study involved two cohorts from the ICU: COVID-19 patients admitted between March 2020 and April 2021 and non-COVID-19 patients from April 2016 to July 2019.
Sample size
The sample size for this retrospective cohort study was determined by including all ICU patients meeting the predefined eligibility criteria during the study periods. To confirm the adequacy of the sample size, a post hoc power analysis was performed based on the observed difference in MAKE-365 incidence (66% vs 39%). A minimum of 554 patients would have been required to detect this difference with a power of 0.9 and an alpha of 0.01, which was exceeded with the inclusion of 708 patients, supporting the robustness of the findings.
Outcomes and definition
The primary outcome of this study was the incidence of MAKE-365 after ICU admission in non-COVID-19 versus COVID-19-ARDS. Secondary outcomes included major adverse kidney events at 30, 90, and 180 days (MAKE-30, MAKE-30, MAKE-90, MAKE-180, respectively), risk factors for MAKE-365 and rates of complete renal recovery at hospital discharge.
MAKE was defined as a composite of receiving new RRT, or reduction in eGFR to <75% of the baseline or all-cause mortality.4 AKI diagnosis and complete renal recovery assessment (eGFR at discharge ≥60 mL/min/1.73 m2) were determined based on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.9
Data collection
Retrospective data collected included patient demographics (age, sex, body mass index (BMI), ethnicity, pre-existing comorbidities, date of ICU admission and date of ICU and hospital discharge), comorbidities, baseline routine laboratory blood results (creatinine and eGFR, white cell count (WCC) and differential, C-reactive protein (CRP), platelet count, albumin and bilirubin) and observations (need for RRT, PaO2/FiO2 ratio, incidence and stages of AKI and mortality) after ICU admission. Baseline eGFR values at 30-, 90-, 180- and 365-day post-ICU admission were extracted in chronological order to track longitudinal changes in renal function.
If baseline eGFR values were missing, the best (highest) preadmission eGFR within the 6 months prior to ICU admission was extracted. If preadmission eGFR was also missing, the baseline eGFR value was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula, due to its greater accuracy in estimating eGFR in diverse populations. This method is recommended by KDIGO guidelines.9 For follow-up eGFR values, the closest values to the specific time points (30, 90, 180 and 365 days from ICU admission) were extracted when the exact values were missing. If there were significant gaps between available values, the closest available value within a specific window (eg, 30 days) was used, or it was recorded as missing if no value fell within this window. Data on new RRT use and mortality at 30, 90, 180 and 365 days from ICU admission were extracted retrospectively from the electronic patient record.
Statistical analysis
All statistical analyses were conducted using R statistical software (V.4.1.2; R Foundation for Statistical Computing, Vienna, Austria). Baseline characteristics were reported as frequencies and percentages for categorical variables. Data distribution was assessed using the Shapiro-Wilk test. Continuous variables, being non-normally distributed, were presented as medians with IQR. Categorical variables were compared using the χ2 test or Fisher’s exact test. For non-parametric data analyses between two groups, the Wilcoxon rank-sum test (independent samples) or Wilcoxon signed-rank test (related samples) was used. The Kruskal-Wallis test was used for comparisons among more than two groups.
Univariate and multivariate logistic regression analyses determined associations between outcomes and parameters, reported as OR with 95% CI. Variables significant in the univariate analysis were included in a backward stepwise multivariable logistic regression model. This selection method was employed due to its efficiency in reducing the model to the most statistically relevant predictors while avoiding overfitting and preserving interpretability. The decision was made to balance statistical rigour with clinical relevance, aligning with standard practices for observational cohort studies. To assess potential multicollinearity and interaction effects, variance inflation factor testing was conducted among the risk factors. No significant multicollinearity or interaction effects were identified, supporting the stability of the final model. The Kaplan-Meier curves plotted time-to-event data for each MAKE-365 component, and differences were analysed using the log-rank test. A p value of <0.05 was considered statistically significant.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
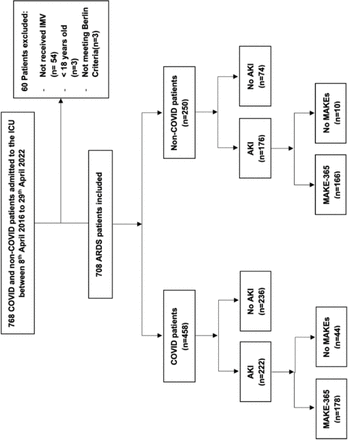
768 ICU admissions within the study duration were screened for eligibility (figure 1). 708 patients were eligible, 458 with COVID-19 and 250 admitted to the ICU before the COVID-19 pandemic; all met the ARDS diagnostic criteria after ICU admission. ARDS aetiology in the non-COVID-19 cohort was further categorised based on the underlying cause of lung injury: 200 patients were identified with ARDS resulting from direct lung injury—such as pneumonia (bacterial, viral, fungal), acute bronchitis and exacerbation of bronchiectasis. The remaining 50 patients were classified as having ARDS from an indirect lung injury, which included cases associated with systemic inflammatory responses such as septic shock and septicaemia without a direct pulmonary source.
Modified CONSORT diagram. This diagram illustrates the process of participant screening and enrolment for the study. A total of 768 ICU patients were screened during the study period, of which 708 were included based on the eligibility criteria for ARDS, COVID-19 status and IMV requirements. The remaining patients were excluded for the following reasons: not receiving IMV (n=54), age <18 years (n=3) or not meeting the Berlin criteria for ARDS (n=3). AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IMV, invasive mechanical ventilation; MAKEs, major adverse kidney events.
Characteristics of acute respiratory distress syndrome patients in COVID-19 versus non-COVID-19 groups
The non-COVID-19 group had a higher median age (61 vs 57 years, p<0.001) and BMI (30 vs 26 kg/m², p<0.001) compared with the COVID-19 group. COVID-19 patients showed higher rates of obesity, diabetes and asthma, while non-COVID-19 patients had more ischaemic heart disease and cancer. CKD rates were similar between groups (table 1). The non-COVID-19 group had higher baseline WCC, CRP and serum creatinine levels (p<0.001), whereas COVID-19 patients had lower albumin and higher platelet counts (p<0.001) (table 1).
The demographic and clinical characteristics of COVID-19 versus non-COVID-19 groups
On admission, the median eGFR was significantly different between COVID-19 and non-COVID-19 groups (90 vs 70 mL/min per 1.73 m², p<0.001). AKI rates were higher in non-COVID-19 patients (70% vs 48%, p<0.001). The non-COVID-19 group had higher RRT use, shorter ICU stay and higher mortality (p<0.001). Overall, there were significantly more patients with moderate (43%) and severe (45%) ARDS compared with mild ARDS (11%), according to the Berlin criteria (p=0.004 and p=0.016, respectively). The distribution of ARDS severities differed significantly between groups such that mild ARDS was less likely seen in COVID-19 patients.
The incidence of major adverse kidney events
The non-COVID-19 group had a higher incidence of MAKE-365 (66% vs 39%, p<0.001), as well as MAKE-30, MAKE-90 and MAKE-180 compared with the COVID-19 group (all p<0.001), predominantly driven by RRT requirements and mortality (table 2). In the non-COVID-19 group, RRT initiation and eGFR decline were observed at higher rates than in the COVID-19 group, particularly at earlier time points (MAKE-30 and MAKE-90). The detailed breakdown of MAKEs by their components, including mortality, eGFR decline and RRT requirements, is provided in table 2.
The incidence of MAKEs outcome and cause of MAKEs in COVID-19 versus non-COVID-19 groups
To explore the non-mortality drivers of MAKE outcomes, additional analysis was conducted, excluding mortality from the composite endpoint (table 3). This revealed that RRT and eGFR decline continued to show higher incidences in the non-COVID-19 group at all time points (MAKE-30, MAKE-90, MAKE-180 and MAKE-365), with statistically significant differences for most comparisons.
Incidence of MAKE outcomes excluding mortality in COVID-19 versus non-COVID-19 groups
Factors associated with major adverse kidney events
In the COVID-19 group, univariate analysis revealed that male sex, older age, Black ethnicity, hypertension, diabetes, ischaemic heart disease, lower eGFR, lower albumin and higher bilirubin levels were associated with MAKE-365 (table 4, figure 2A), as well as MAKE-30 (online supplemental tablet 1), MAKE-90 (online supplemental table 2) and MAKE-180 (online supplemental table 3). In multivariable analysis, older age, diabetes, lower eGFR and higher bilirubin levels were significant predictors across all MAKE outcomes, including MAKE-30, MAKE-90, MAKE-180 and MAKE-365.
Supplemental material
Binomial logistic regression analysis of factors associated with MAKE-365 in COVID-19 versus non-COVID-19 groups
Forest plots of factors associated with major adverse kidney event at 365 days (MAKE-365) in COVID-19 and non-COVID-19 acute respiratory distress syndrome (ARDS) groups. Forest plots illustrating factors associated with MAKE-365 in (A) COVID-19 and (B) non-COVID-19 ARDS groups, based on multivariable logistic regression analysis. ORs with 95% CI are displayed. Significant predictors in the COVID-19 group (A) include older age, diabetes, lower eGFR and higher bilirubin levels, while significant predictors in the non-COVID-19 group (B) include lower eGFR, lower albumin and higher bilirubin levels. eGFR, estimated glomerular filtration rate.
In the non-COVID-19 group, univariate analysis indicated that lower WCC, lower eGFR, lower albumin and higher bilirubin levels were associated with MAKE-365 (table 4, figure 2B), MAKE-30 (online supplemental table 1), MAKE-90 (online supplemental table 2) and MAKE-180 (online supplemental table 3). Multivariable analysis identified lower eGFR, lower albumin and higher bilirubin levels as consistent independent predictors of MAKE-30, MAKE-90, MAKE-180 and MAKE-365 development.
Time to event of individual major adverse kidney event outcome
The Kaplan-Meier curves for time-to-event data for each MAKE component at 365 days from ICU admission among patients with MAKE-365 in the COVID-19 and non-COVID-19 groups are displayed in figure 3.
The Kaplan-Meier curves for time to event of individual MAKE outcome. These Kaplan-Meier curves illustrate no difference in the initiation of RRT (A) for patients with MAKE-365 across both groups. However, the non-COVID-19 group demonstrated a higher risk of eGFR decline (B) and decreased 1-year survival (C) compared with the COVID-19 group. eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy.
Renal replacement therapy
The Kaplan-Meier analysis revealed no significant difference in the time to initiate RRT among patients with MAKE-365 in both groups, with RRT initiation commonly observed within the first 30 days following ICU admission (p=0.53, log-rank test). The Cox proportional hazards model further confirmed this finding, with no significant difference in risk between the two groups (HR=0.943, 95% CI: 0.737 to 1.207, p=0.643).
Estimated glomerular filtration rate decline (<75% of baseline)
Patients in the non-COVID-19 group had a significantly higher risk of eGFR decline compared with the COVID-19 group over the 365-day follow-up period (p=0.006, log-rank test). The Cox proportional hazards model supported this observation with an HR of 0.659 (95% CI: 0.490 to 0.888, p=0.006), indicating a lower risk of eGFR decline in the COVID-19 cohort.
Mortality
A 1-year survival was significantly lower in the non-COVID-19 group compared with the COVID-19 group (p=0.005, log-rank test). The Cox proportional hazards model indicated a significantly higher risk of mortality in the non-COVID-19 group (HR=1.457, 95% CI: 1.114 to 1.905, p=0.006).
Estimated glomerular filtration rate and mortality after initiating renal replacement therapy
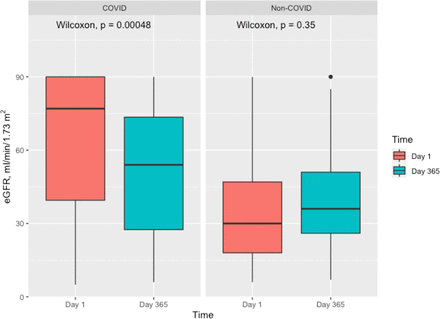
Patients requiring RRT earlier in admission showed worse renal outcomes and survival rates than those who did not. Initiation of RRT within 30 days was significantly associated with lower eGFR at 1 year and higher mortality in both COVID-19 (online supplemental table 4) and non-COVID-19 groups (online supplemental table 5). In the COVID-19 group, patients who received RRT within 30 days and completed 1-year follow-up had a significant decrease in the median eGFR by 365 days compared with their eGFR on ICU admission (p=0.00048), while there was no significant difference in median eGFR in non-COVID-19 patients (p=0.35), as displayed in figure 4.
Comparison of Median eGFR on day 1 and day 365 following ICU admission in patients who received RRT within 30 days. This figure illustrates a significant difference in the median eGFR by 365 days compared with their eGFR on ICU admission, while there was no significant difference in median eGFR in non-COVID-19 patients. eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy.
Renal recovery
There were no significant differences between the COVID-19 and non-COVID-19 groups in the proportion of AKI patients who achieved complete renal recovery at hospital discharge, as assessed at 30, 90 and 180 days following ICU admission (online supplemental table 6).
Incidence of acute kidney injury and major adverse kidney event at 365 days across acute respiratory distress syndrome severities
In the COVID-19 group (n=458), there were no statistically significant differences in the incidence of AKI and MAKE-365 across ARDS severities (p=0.276 and p=0.17, respectively) (online supplemental table 7), although there was a trend of higher incidences of MAKE-365 in the moderate to severe ARDS categories compared with mild ARDS. In contrast, the non-COVID-19 group showed a significant difference in the incidence of AKI (p=0.032) and MAKE-365 (p=0.027) across ARDS severity cohorts (online supplemental table 8). These data indicate that in the non-COVID-19 ARDS population, there is a stronger association between ARDS severity and the incidences of AKI and MAKE-365.
Incidence of major adverse kidney event among acute respiratory distress syndrome patients with mainly respiratory infection
The incidence of MAKE outcomes at 30, 90, 180 and 365 days after ICU admission among ARDS patients with only respiratory infection is shown in online supplemental table 9. This analysis included 658 patients, of which 458 were in the COVID-19 group and 200 in the non-COVID-19 group. The incidence of MAKE-365 was higher in the non-COVID-19 group (38.9% vs 61.0%, p<0.001).
Discussion
Our study fills the gap in understanding long-term kidney outcomes for ICU admissions with ARDS, comparing COVID-19 and non-COVID-19 patients. Non-COVID-19 ARDS patients had higher incidences of AKI, RRT and MAKE-365. The primary drivers were RRT initiation, mortality and eGFR decline. The higher incidence of adverse outcomes in the non-COVID-19 cohort can be attributed to both baseline population differences and disease-specific factors. Non-COVID-19 patients presented with a greater burden of comorbidities, including older age, ischaemic heart disease and cancer, which may have increased the risk of renal complications and mortality.10 Additionally, non-COVID-19 ARDS often involves a heterogeneous mix of respiratory failure causes, such as bacterial pneumonia and sepsis, which are known to trigger multi-organ failure, whereas COVID-19 ARDS typically presents with more isolated viral pneumonia.11
MAKE incidence increased over time (30, 90, 180 and 365 days) in both groups, typical for cumulative outcomes. The higher MAKE-365 incidence in the non-COVID-19 group suggests a more severe long-term ARDS impact, aligning with Vinclair and De Montmollin,12 who reported high MAKE incidences in venoarterial extracorporeal membrane oxygenation (VA-ECMO) patients.
It has been well-known that AKI contributes to higher rates of morbidity and mortality, especially in patients with ARDS.13 Our study found greater AKI incidence and severity in the non-COVID-19 group, consistent with Panitchote and Mehkri.14 Proposed mechanisms for AKI onset during ARDS include haemodynamic, metabolic and neurohormonal imbalances, which have been extensively described in previous literature.15 16
ARDS and IMV remain independent risk factors for AKI, alongside older age, diabetes and cardiac dysfunction.17 However, the observed differences in kidney outcomes between COVID-19 and non-COVID-19 ARDS patients may also reflect disease-specific pathology. COVID-19 ARDS is often characterised by viral pneumonitis with a lower incidence of superimposed bacterial infections, whereas non-COVID-19 ARDS frequently involves bacterial sepsis and systemic inflammation, both of which are known to worsen renal outcomes and contribute to multi-organ failure.18 Management strategies like lower oxygen saturation tolerance and permissive hypercapnia increase diuresis and renal resistive indices, potentially reducing glomerular filtration rate (GFR) and leading to AKI.19
In the present study, the non-COVID-19 group, characterised by older age, exhibited a higher prevalence of pre-existing comorbidities (including ischaemic heart disease and cancer), elevated baseline serum creatinine levels, decreased eGFR and elevated counts of circulating leucocytes and neutrophils. While elevated leucocyte counts indicate an inflammatory response rather than directly indicating kidney dysfunction, these findings suggest potential underlying health differences that could affect renal outcomes. This aligns with findings from a prior study comparing characteristics and outcomes of ARDS patients with and without COVID-19.11 These differences underscore the complex interaction between pre-existing health status, severity of illness and renal outcomes, which likely contributed to the higher rates of MAKE-365 observed in the non-COVID-19 cohort.
There is limited published data investigating the risk factors for MAKE-365 in COVID-19-related ARDS. The present study found that older age, diabetes, reduced eGFR and elevated bilirubin levels were strongly associated with a higher risk of MAKE-365 in the COVID-19 group. However, we acknowledge that baseline differences in population characteristics, including comorbidities and severity of illness at ICU admission, may have influenced these associations and should be considered when interpreting the findings. A recently published study demonstrated that COVID-19 patients with high direct bilirubin levels had higher risks of shock and respiratory failure during hospitalisation.20 Elevated bilirubin levels may reflect underlying hepatic injury or systemic inflammation, both of which can contribute to the severity of illness in COVID-19 patients, potentially leading to worse outcomes such as ARDS and multiple organ failure.21
Our findings align with recent reports, such as Aklilu et al7, who noted a lower incidence of MAKE in COVID-19 AKI patients compared with those with AKI from other illnesses.7 This suggests that long-term kidney outcomes in COVID-19 survivors may be more favourable than initially anticipated.
RRT initiation was higher in the non-COVID-19 group, consistent with current literature.22 23 This may be due to different pathophysiological presentations, with COVID-19 ARDS patients often presenting single-organ failure, while non-COVID-19 patients frequently presented with multi-organ failure, increasing renal impairment risk.11 Resource limitations during the pandemic may have led to a more cautious ‘wait-and-see’ approach to RRT initiation; however, all patients meeting clinical criteria ultimately received RRT, and the impact of delayed initiation on outcomes was minimised under the START-AKI protocol.
Lumlertgul and Pirondini24 found that 32% of COVID-19 patients received RRT early in ICU stay. Indications included high urea, oliguria, hyperkalaemia, acidosis, pulmonary oedema, rhabdomyolysis and fluid/electrolyte imbalances.24 A common rationale while treating ARDS patients with AKI is that early initiation of RRT prevents fluid overload and uraemia, thus promoting a faster recovery.25 However, in contrast with the previous study, we found that patients who had RRT within 30 days were at a higher risk of mortality by 365 days in both groups. It is important to note that these patients also had lower baseline eGFRs. Therefore, it appears that it is the severity of the AKI, rather than the RRT itself, that increases the mortality risk. The recent STARRT-AKI study supports this view, showing that accelerated RRT was associated with worsened longer-term renal outcomes, particularly in patients with reduced eGFR.26
We found a higher incidence of MAKE-365 in non-COVID-19 ARDS patients, possibly due to several factors. Non-COVID-19 respiratory infections may lead to multi-organ failure, increasing renal impairment risk.11 Su et al27 reported that kidney disease was associated with higher mortality in non-COVID-19 respiratory infections. A higher burden of comorbidities and different patient demographics during admission may also contribute to increased MAKE-365 incidence.
The present study assessed the renal recovery of AKI patients at hospital discharge and found no significant differences among those who had complete renal recovery in the two groups at 30, 90 and 180 days. Interestingly, the number of patients who had completed renal recovery and received RRT in the COVID-19 group increased over time. This observation may be related to the unique pathophysiology and treatment protocols for COVID-19 patients. Future research should explore the factors contributing to the increased use of RRT in COVID-19 patients who initially achieve renal recovery.
The findings of this study emphasise the importance of MAKE-365 as a composite endpoint to assess long-term kidney health following ARDS. Specifically, the results highlight the need for targeted clinical interventions in high-risk groups, such as patients with reduced eGFR and elevated bilirubin levels, both of which were independent predictors of MAKE-365 in this study. Closer monitoring of kidney function, earlier intervention strategies for renal protection and individualised care plans based on baseline risk factors may help improve long-term outcomes. Additionally, MAKE-365 could serve as a useful prognostic tool in the development of post-ICU discharge care pathways, identifying patients who may benefit from nephrology follow-up and chronic kidney disease screening. Future clinical trials should explore whether early detection and management of MAKE components could reduce long-term morbidity and mortality in ARDS survivors.
Our study’s strengths include a large sample size, consistent management and lengthy follow-up, enhancing the reliability and relevance of our findings. However, several limitations should be acknowledged. The study was conducted at a single centre, which may limit the generalisability of the findings. Reliance on preadmission eGFR estimates may have introduced variability in baseline kidney function assessment. Additionally, the lack of competing risk analysis could have influenced the interpretation of long-term kidney outcomes. The unequal sample sizes between the COVID-19 (n=458) and non-COVID-19 (n=250) cohorts could influence the statistical power and contribute to baseline differences between the groups. However, all available eligible patients meeting the inclusion criteria during the study periods were included, and adjustments for key baseline characteristics were made in the multivariable analyses to account for this imbalance.
The use of the MAKE-365 composite endpoint, while aligned with internationally recognised nephrology definitions,4 reflects a cumulative outcome and may not fully capture dynamic changes in renal function over time, such as resolved kidney injury or new-onset dysfunction during follow-up. This could obscure the distinction between persistent kidney injury and recovered renal function. To address this concern, we have presented the individual MAKE components (RRT, reduced eGFR and mortality) separately in table 2 to clarify the specific drivers of MAKE incidence. Differences in ICU admission criteria and resource constraints during the COVID-19 pandemic may have further contributed to selection bias. Although all patients meeting clinical criteria ultimately received RRT, a more cautious ‘wait-and-see’ approach was sometimes applied, as described under the STARRT-AKI protocol.
Additionally, the inclusion of patients from multiple hospitals across the Midlands region led to some loss to follow-up due to variations in electronic health record systems and patient transfers between institutions, which limited the ability to consistently capture long-term follow-up data. Furthermore, the lengthy ICU stays and muscle mass loss observed in some patients may have led to an overestimation of renal recovery due to suppressed creatinine levels. Despite these limitations, our findings provide important insights into long-term kidney outcomes in critically ill ARDS patients and emphasise the need for further research using more dynamic renal recovery metrics and competing risk models to fully capture the complexity of kidney function changes over time.
In summary, MAKE-365 was more prevalent in non-COVID-19 ARDS patients, primarily driven by higher RRT initiation and mortality rates. Key risk factors included lower eGFR and elevated bilirubin in both groups, with additional contributions from age and diabetes in the COVID-19 cohort, and lower albumin levels in the non-COVID-19 group. These findings emphasise the need for continued research on adverse kidney events in ARDS patients, with a focus on targeted interventions for high-risk groups. Future strategies could explore developing a risk score system to better predict MAKE-365 and improve long-term outcomes.
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request. The de-identified participant datasets analysed for this study are available on reasonable request from the corresponding author at d.parekh@bham.ac.uk.
Ethics statements
Patient consent for publication
Ethics approval
Ethical approvals for non-COVID-19 ARDS patients were obtained from the Wales Research and Ethics Committee 1 (REC 16/WA/0169) under the AM-ARDS study, with subsequent approval by the Health Research Authority (HRA) and University Hospitals 24 Birmingham and Heart of England NHS Trusts (NIHR Portfolio, registration number 32296). During the COVID-19 pandemic, the HRA waived the need for additional ethical approval and consent for observational studies involving COVID-19 patients (https://www.hra.nhs.uk/covid-19-research/guidance-using-patient-data/). Local governance approval was granted by the audit and quality improvement departments for the collection of observational data from patients with COVID-19.
References
Footnotes
X @drdhruvparekh
Contributors FKA was responsible for study conceptualisation, methodology, data analysis, visualisation and manuscript preparation. JP, DT and MNB contributed to study planning, design and critical manuscript revision. RYM and DP were involved in data collection, result interpretation and critical manuscript review. FKA is the guarantor for the study and takes full responsibility for the content of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.