Article Text
Abstract
Introduction Recently, immunotherapy has significantly transformed the treatment landscape of endometrial cancer (EC). Results from KEYNOTE-158, RUBY and AtTEnd showed programmed cell death 1 (PD-1) or programmed cell death-ligand 1 inhibitors with promising efficacy in primary advanced or recurrent EC. However, few studies focused on the role of dual immune checkpoints in primary advanced or recurrent EC. Cadonilimab is an immune checkpoint inhibitor targeting the PD-1 and T-lymphocyte antigen-4, which is expected to show substantial clinical efficacy in EC. Combining cadonilimab with standard chemotherapy may have synergistic effects, making this combination a promising first-line treatment for primary advanced or recurrent EC. Furthermore, incorporating molecular classification for guidance on the use of cadonilimab may hold valuable clinical benefits.
Methods and analysis In this multicentre, open-label, phase II study, patients with histologically confirmed EC were eligible. Forty-five patients will be recruited. Seventeen patients will be enrolled in stage I, and at least seven cases of complete response (CR) and partial response (PR) should be observed before entering stage II. All patients will receive cadonilimab at a dosage of 10 mg/kg along with carboplatin (area under the curve (AUC)=4–5) plus paclitaxel (175 mg/m2) every 3 weeks (Q3W) for 6–8 cycles. Subsequently, patients with CR, PR or stable disease will receive maintenance of cadonilimab at 10 mg/kg Q3W for 24 months or until progressive disease or adverse events are reported. The objective response rate is the primary endpoint. The secondary endpoints include the disease control rate, duration of response, progression-free survival, overall survival and safety. Additionally, exploratory endpoints involve biomarkers that may predict the efficacy of cadonilimab and chemotherapy, as well as their relationship with molecular classifications. The interim analysis will be conducted after 17 patients have been enrolled.
Ethics and dissemination The study protocol meets the approval of the ethical committee of Fujian Cancer Hospital (K2023-173-04) and all other participating hospitals. Study findings will be disseminated in peer-reviewed publications.
Trial registration number NCT06066216.
- Gynaecological oncology
- CHEMOTHERAPY
- IMMUNOLOGY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This study is the first prospective investigator-initiated trial to evaluate the efficacy of cadonilimab combined with chemotherapy as the first-line therapy for primary advanced or recurrent endometrial cancer. It also examines the efficacy of cadonilimab across different molecular classifications.
This trial is a multicentre, open-label, phase II study with a Chinese cohort of 45 patients.
Objective response rate is the primary endpoint. Disease control rate, duration of response, progression-free survival, overall survival and adverse events were also assessed.
Single-arm design may be biased regarding patient selection and outcome evaluation.
Introduction
In the discipline of gynaecological oncology, endometrial cancer (EC) is one of only a few female malignancies for which both incidence and mortality are constantly rising.1 2 According to the Global Cancer Statistics 2022, there were 420 242 new cases, with fatalities numbering 97 704, representing a substantial economic and health burden.3 The Global Cancer Observatory estimated that China accounted for 18.49% of all new EC cases and 13.8% of EC-related deaths globally in 2022.4 Recent data indicate that the mortality rate for EC has been rising more quickly than the incidence rate. This rise in mortality may be due to an increase in advanced-stage and recurrent cases, considering patients with EC are often detected and successfully treated at an early stage, resulting in favourable outcomes.5 Patients with advanced or recurrent EC have a poor prognosis, with a median survival of 12–18 months.6 For many years, the regime of combining paclitaxel and carboplatin has been the standard chemotherapy for the treatment of primary advanced or recurrent EC. However, outcomes still remain stagnant and dismal, with a 5-year survival rate of 17%.7 The primary challenge lies in developing a more effective combined regimen with less toxicity for advanced-stage and recurrent EC.
Recently, outstanding results from KEYNOTE-158,8 GARNET,9 RUBY10 and NRG-GY0182 trials confirmed the use of immunotherapy, such as dostarlimab and pembrolizumab, in cytotoxic chemotherapy for advanced and metastatic EC with encouraging improved survival outcomes, especially in the deficiency mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) population. However, most advanced or recurrent EC tumours are proficient in mismatch repair (pMMR), comprising approximately 75% of cases. This underscores the necessity for further investigation and the discovery of well-tolerated treatments for managing advanced or recurrent cases, including pMMR. Immunotherapy aims to boost natural defences to eradicate cancer cells.11 Monoclonal antibody therapies, such as dostarlimab and pembrolizumab mentioned above, target the interaction between programmed cell death-1 (PD-1) and its ligands and could increase the T-cell activity and avoid immune escape to destroy cancer cells.12 A new era is dawning where we are starting to see more promising and effective immunotherapy drugs or a better myriad of therapeutic agents, with fewer adverse events (AEs), in primary advanced or recurrent EC, such as LEAP-001,13 AtTEnd14 and DUO-E15 trials. However, antibodies blocking PD-1/PD-L1 could upregulate the expression of anticytotoxic T-lymphocyte antigen-4 (CTLA-4) and inhibit T-cell activity, leading to the limited efficacy of monotherapy against PD-1/programmed cell death-ligand 1 (PD-L1).16 Cumulative results from pivotal phase III trials also confirmed higher response rates of the combination of the anti-PD-1 antibody and anti-CTLA-4 antibody than anti-PD-1 monotherapy in various late-stage cancers, such as melanoma,17 non-small-cell lung cancer18 and gastro-oesophageal cancer.19 However, incidences of treatment-related AEs and toxicities limit the broad application of this combination.
Cadonilimab is a new high-density bispecific PD-1/CTLA-4 antibody with a more robust ability in active T cells and was well-tolerated with minimal cellular cytotoxicity.20 A growing body of evidence of ongoing clinical trials and case reports supports the use of cadonilimab in various cancers,21–24 such as advanced non-small-cell lung cancer, recurrent or metastatic cervical cancer and advanced gastric cancer. Recently, Lan et al reported promising antitumour activity of cadonilimab plus lenvatinib with an objective response rate (ORR) of 42.9% (12/28) in patients with advanced EC who experienced disease progression after prior systemic platinum-based therapy.25 Hence, the above findings provide a rationale to test cadonilimab, along with standard chemotherapy, in an effort to develop a more effective treatment regimen with less toxicity in advanced or recurrent EC.
The current clinical trial designs for EC do not fully incorporate molecular classification. Most trials only stratify patients according to MMR status. Preclinical and clinical investigations suggested that dMMR/MSI-H was found to be attractive with the treatment of monoclonal antibody therapies against PD-1 and PD-L1 in advanced or recurrent EC, while pMMR type demonstrated less efficacy2 26 27 which might be explained by dMMR/MSI-H’s tumour microenvironment features of increasing PD-1+CD8+ T-cell infiltration and the highest PD-L1 expression in tumour- and immune-infiltrating cells.28 Subgroup analysis from the RUBY trial showed that the combination of dostarlimab and carboplatin/paclitaxel demonstrated clinical benefits in the dMMR/MSI-H, TP53 (Tumor protein 53)-mutated and NSMP (no specific molecular profile) molecular subgroups.29 This is consistent with findings from the Lan study, which reported an ORR of 33.3% in the dMMR/MSI-H group, 50.0% in the NSMP group and 16.7% in the TP53 abnormal group.25 With more robust immune activation power, we believe cadonilimab could work more effectively for pMMR or anti-PD-1-nonresponsive type. Hopes are pinned on molecular classification-guided therapies in cadonilimab to combat immunotherapy resistance or enhance response to ICIs (Immune checkpoint inhibitors).
This phase II trial investigates the efficacy and safety of cadonilimab in patients with primary advanced or recurrent EC. Additionally, the trial seeks to answer the important question of whether cadonilimab could play a favourable role in other molecular subtypes beyond the dMMR.
Methods/design
Objectives
The primary objective is to evaluate the efficacy of cadonilimab combined with paclitaxel and carboplatin as the first-line treatment for patients with primary advanced or recurrent EC. The secondary objective is to assess the safety of this combination treatment. We also explore response rates of cadonilimab and chemotherapy in EC across different molecular characteristics. We hope EC molecular characteristics can shed new light on guiding the use of cadonilimab for precision medicine.
Study design
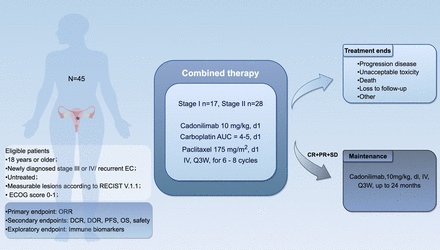
The study aims to enrol 45 patients, with enrolment starting in December 2023 and concluding in December 2025. Seventeen patients will be enrolled in stage I, with at least seven patients achieving complete response (CR) and partial response (PR) before entering stage II. Treatment-naive tumour tissue will be obtained to confirm molecular classification and PD-L1 level. Blood samples will also be collected. All patients will receive cadonilimab and chemotherapy of carboplatin plus paclitaxel every 3 weeks for 6–8 cycles. Patients with CR, PR or stable disease (SD) will receive cadonilimab maintenance for 2 years or until progressive disease (PD) or unbearable AEs are discovered (figure 1).
The trial design. AUC, area under curve; CR, complete response; DCR, disease control rate; DOR, duration of response; EC, endometrial cancer; ECOG, Eastern Cooperative Oncology Group; IV, intravenous; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; Q3W, every 3 weeks; SD, stable disease
Study endpoints
The primary endpoint is the ORR, defined as the proportion of patients who achieve a CR or PR according to Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1.30 Other key secondary and exploratory endpoints are listed in box 1.
Study endpoints
Primary endpoint
ORR
Secondary endpoints
DCR
DOR
PFS as determined by the investigator
OS
OS rate at 12 and 24 months from the first drug administration
AEs such as nausea, alopecia, fatigue, rash, anaemia, neutropaenia and infection
Exploratory endpoints
ORR in patients with different molecular classification
Relationship between cadonilimab and molecular classification
AEs, adverse events; DCR, disease control rate; DOR, duration of response; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Key eligibility criteria
Key patient eligibility criteria are listed in box 2.
Patient inclusion and exclusion
Inclusion criteria
18 years or older.
Newly diagnosed stage III or IV/recurrent cases with histologically confirmed EC, including endometrioid adenocarcinoma, serous adenocarcinoma, clear cell adenocarcinoma, mixed epithelial carcinoma, etc.
Without systemic therapy at the time of the enrolment, the prior antiangiogenic treatment is permitted.
Unresectable and measurable lesions could be defined and monitored by RECIST V.1.1.
Adequate organ function and ECOG of 0–1.
Written informed consent.
Life expectancy >3 months.
Exclusion criteria
History of other malignancies within 5 years or simultaneously.
Immunodeficiency disease or long-term use of immunosuppressive agents.
Uncontrolled infection, which needs systemic therapy.
Serious medical illness, such as severe mental disorders, cardiac disease, any arterial/venous thromboembolic event, transient cerebral ischaemia, hypertensive crisis, severe bleeding, coagulation disorders, and digestive system diseases such as ulcers, abdominal fistula and tumour-related gastrointestinal obstruction.
Unqualified of baseline blood and biochemical indicators.
Any previous or current disease, treatment or laboratory test abnormality that may confound the study endpoints.
ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors.
Interventions
All participants in the study will undergo 6–8 cycles of treatment consisting of cadonilimab along with paclitaxel and carboplatin. Patients who exhibit CR, PR or SD will receive cadonilimab maintenance for 24 months. The interventions will be stopped if the investigator determines that continuance would pose a significant safety risk. The details are shown as follows.
Immunotherapy
All subjects will receive cadonilimab at a dosage of 10 mg/kg intravenously on day 1, administered over 120 min every 3 weeks for 6–8 cycles.
Chemotherapy
All subjects will be given the standard regimen for primary advanced or recurrent EC, including carboplatin (area under the curve (AUC)=4–5, on day 1) and paclitaxel (175 mg/m2, on day 1) every 3 weeks for 6–8 cycles. If patients are allergic to carboplatin, cisplatin will be administered at a dose of 75 mg/m2. The investigators will determine the detailed treatment courses.
Maintenance
After finishing the 6–8 treatment cycles, patients with CR, PR or SD will continue to receive cadonilimab at 10 mg/kg on day 1, every 3 weeks, for up to 24 months or until the discovery of PD or intolerant AEs.
Evaluations
Subjects will undergo tumour assessments by CT or MRI every 6 weeks within 24 weeks, then every 9 weeks during 25–51 weeks and every 12 weeks after 52 weeks until radiological progression, loss of follow-up, death, withdrawal of informed consent or end of the study (figure 2). CR or PR is confirmed by the RECIST (V.1.1) based on radiological changes.30 Disease control rate (DCR) is assessed as the sum of CR, PR and SD. Duration of response (DOR) is the date of first documented PR/CR to the date of PD or death. Progression-free survival (PFS) is defined as the time from the first dose to the date of objective disease progression or death. Overall survival (OS) is calculated from the date of the first dose until death due to any cause. In addition, we conduct and compare subgroup analyses of ORR, DOR, DCR, PFS and OS under different molecular classifications.
Summary of patient timeline and assessments. AE, adverse event; ECOG, Eastern Cooperative Oncology Group; IHC, immunohistochemistry; NGS, next generation sequencing; PD-1, programmed cell death 1; PD-L1, programmed cell death-ligand 1.
Sample size
The sample size is calculated according to the expected rate of the primary endpoint efficacy and the anticipated size of the effect of this combination regime. Based on the previous research of 40% ORR,31 the response rate of this trial is set to be 60%. The minimum study sample size is required to obtain at least 80% power with a one-sided significance level of 0.05 (alpha). With the assumption of a 10% dropout rate, the planned sample size is 45. Using the Simon optimal two-stage design, 17 cases were included in stage I. If seven or fewer patients have a response, the study is terminated. Otherwise, the study will continue to stage II, where 28 patients will be recruited.
Statistical methods
All statistical analyses will be performed using SPSS V.22.0. For continuous variables, the IQR and mean±SD will be used, and comparisons will be made using the t-test. Categorical variables will be represented using percentages and numbers, and comparisons will be made using the χ2 test. Survival analysis will be conducted using the Kaplan-Meier method and log-rank test analysis. Prognostic factors will be identified using Cox proportional hazards analysis. A p value of <0.05 will be considered significant.
Patient and public involvement
There was no involvement of patients or the public in this study’s research design and conception.
Ethics and dissemination
The study protocol meets the approval of the ethical committee of Fujian Cancer Hospital (K2023-173-04) and all other participating hospitals. The trial results will be disclosed and evaluated by scientific journals and international conferences.
Discussion
Immunotherapy with checkpoint inhibitors has revolutionised the treatment paradigm for many tumours, and EC is no exception. This phase II trial investigates the efficacy and safety of cadonilimab in combination with standard chemotherapy in the first-line setting for patients with primary advanced or recurrent EC, which could potentially usher in a new era of EC management. Additionally, understanding cadonilimab’s effectiveness with different molecular classifications may significantly advance tailored treatment approaches for patients with primary advanced or recurrent EC.
Numerous PD-1/PD-L1 and CTLA-4 blockers have been developed for better cancer treatment. A combination of different immunotherapy regimens with PD-1 and CTLA-4 blocks has synergistic effects. Cadonilimab is a tetravalent bispecific antibody with a combination of PD-1/CTLA-4 blockers and Fc-null region, exhibiting high-binding avidity.20 PD-1 pathway blockade could keep the activity of antitumour T cells, which should have become quiescent. CTLA-4 blockade could raise and activate more T cells and reduce immunosuppression, which was mediated by Treg cells. A blockade of dual pathways could work together, resulting in a more significant, long-lasting antitumour immune response (figure 3). In addition, the Fc-null part could enhance antitumour activity by inhibiting the binding of the Fc receptor, leading to the elimination of antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity and cytokine secretion. Moreover, such enhanced binding avidity of cadonilimab prefers to accumulate in the tumour microenvironment with a high density of both antigens, not in normal peripheral tissues, thus amplifying efficacy but avoiding toxicity.32 Recent clinical trials showed that cadonilimab monotherapy exhibits manageable AEs with an incidence of ≥grade 3 AEs of 12–26%.22 23 33 34 Lan et al also found that grade 3 or 4 treatment-related AEs occurred only in seven (21.9%) patients with advanced EC treated with cadonilimab plus lenvatinib as second-line or later therapy.25 Additionally, a phase II clinical trial (NCT06532539) of cadonilimab combined with chemoradiation for recurrent and oligometastatic endometrial carcinoma is ongoing. Taken together, cadonilimab potentially represents a new promising immunotherapy for EC treatment.
Mechanism and structure of cadonilimab. Anti-PD-1 region: PD-1 pathway blockade could keep the activity of antitumour T cells, which should have become quiescent. Anti-CTLA-4 region: CTLA-4 blockade could raise and activate more T cells and reduce immunosuppression mediated by Treg cells. Anti-CTLA-4, anticytotoxic T-lymphocyte antigen-4; CTLA-4, cytotoxic T-lymphocyte antigen-4; MHC, major histocompatibility complex; PD-1, programmed cell death 1; PD-L1, programmed cell death-ligand 1; TCR, T-cell receptor; Treg, regulatory T cells.
A significant focus is emerging on exploring the synergy of immunotherapy and conventional cancer therapies, as monotherapy fails to meet the treatment needs. Accumulating data highlighted the safety and effectiveness of ICIs in chemotherapy in mouse models and clinical trials.2 7 9 10 35 Chemotherapy could cause initial antitumour responses but is often followed by tumour regrowth, which could be resolved by ICIs. When attacked by T cells, tumour cells might be more susceptible to cytotoxic drugs.36 Cytotoxic drugs could also kill tumour cells that escape T-cell attack, suppress Tregs regulation in immune stimulation and enhance the antigen presentation functions, resulting in more powerful tumour-killing abilities.37 It is also worth noting that peripheral immune cells cannot access the tumour in some circumstances. However, combination chemotherapy can inhibit immune suppressive cells, produce cytokines or chemokines and enhance tumour antigen exposure to the active immune system.37 38 Hence, it is a promising strategy for applying cadonilimab in first-line chemotherapy for patients with primary advanced or recurrent EC.
Currently, molecular classification is increasingly incorporated into clinical EC management.39 Initially proposed by The Cancer Genome Atlas, EC is currently classified into four molecular groups, namely, POLEmut, MMRd, p53abn and NSMP subgroups.40 In clinical settings, dMMR/MSI-H status is proven to be an effective predictive biomarker for immunotherapy sensitivity in patients with EC. Results from the Ruby study showed by adding pembrolizumab to standard chemotherapy, followed by pembrolizumab maintenance, a 70% lower risk of tumour progression or death in dMMR patients and a 46% lower risk in the pMMR subgroups was detected compared with the control group.2 The GARNET trial also demonstrated that dostarlimab exhibited durable antitumour activity with an ORR of 43.5% for dMMR/MSI-H and an ORR of 4.1% for MMRp/MSS EC.26 Since cadonilimab could simultaneously activate PD-1/CTLA-4 pathway blockade, there is sufficient reason to believe cadonilimab could produce a more powerful antitumour immune reaction, even for pMMR or nonresponsive dMMR/MSI-H type. Moreover, whether the molecular classification has predictive value for the benefit of cadonilimab has not yet been investigated. This trial aims not only to illuminate the value of implementing the molecular-based classification for cadonilimab guidance but also to find the novel characterisation of heterogeneous molecular groups for better selection of personalised treatment plans for EC in the late stages.
This trial offers flexibility for patients with advanced or recurrent conditions to access potentially more effective treatments. Additionally, the biomarker analysis plan includes both tumour and blood samples to assess a broad range of biomarkers, including MMR status, TMB (Tumour mutational burden) and PD-L1. However, a limitation of this trial is that it will be a cohort of a small number of Chinese patients, which might result in a risk of underestimating or overestimating the efficacy. We welcome participants of different ethnicities. Second, the single-arm design may be biased regarding patient selection and outcome evaluation. We need strict eligibility criteria, objective endpoints and independent assessment of safety data.
Conclusion and future perspectives
This prospective single-arm, open-label phase II trial aims to provide evidence for the use of cadonilimab in combination with standard chemotherapy as a new therapeutic strategy for first-line treatment of primary advanced or recurrent EC. We believe the findings from this trial could offer valuable insights for future phase III trials testing cadonilimab as a first-line treatment option for primary advanced or recurrent EC. Moreover, exploring the relationship between molecular classification and the effectiveness of cadonilimab will provide much-needed perspective data for directing precise treatment for EC in the late stage. Cadonilimab and its integration with molecular classification will offer new potential therapeutic opportunities in EC management in the coming years.
Ethics statements
Patient consent for publication
Acknowledgments
We appreciate the contribution of patients and collaborators for participating in this study, along with all investigators and site personnel.
References
Footnotes
JL and TL contributed equally.
Contributors YS contributed to the conception of the study. The manuscript protocol was drafted by JL and revised by YS. YS, TL, JC and YingtaoL arbitrated the disagreements and ensured no errors were introduced during the study. YS, JL, XC, YZ, YuzhiL, YJ, LY, CT, BL, JZ and LC assisted in further protocol development. YS is the guarantor. All authors approved the publication of the protocol.
Funding This work was funded by the Natural Science Foundation of Fujian Province of China (Grant number 2024J011098); the Clinical Research Center for Precision Treatment of Gynecological Malignancies of Fujian Province, China (Grant number 2022Y2015) and the Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number 2023Y9404).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.