Article Text
Abstract
Introduction Heart failure with preserved ejection fraction (HFpEF) is common and causes functional limitation, poor health-related quality of life (HRQoL) and impairs prognosis. Exercise-based cardiac rehabilitation is a promising intervention for HFpEF, but there is currently insufficient evidence to support its routine use. This trial will assess the clinical and cost-effectiveness of a 12-week health professional-facilitated, home-based rehabilitation intervention (REACH-HF), in people with HFpEF, for participants and their caregivers.
Methods and analysis REACH-HFpEF is a parallel two group multicentre randomised controlled trial with 1:1 individual allocation to the REACH-HF intervention plus usual care (intervention group) or usual care alone (control group) with a target sample size of 372 participants with HFpEF and their caregivers recruited from secondary care centres in United Kingdom. Outcome assessment and statistical analysis will be performed blinded; outcomes will be assessed at baseline and 4-month and 12-month follow-up. The primary outcome measure will be patients’ disease-specific HRQoL, measured using the Minnesota Living with Heart Failure questionnaire, at 12 months. Secondary outcomes include patient's exercise capacity, psychological well-being, level of physical activity, generic HRQoL, self-management, frailty, blood biomarkers, mortality, hospitalisations, and serious adverse events, and caregiver's HRQoL and burden. A process evaluation and substudy will assess the fidelity of intervention delivery and adherence to the home-based exercise regime and explore potential mediators and moderators of changes in HRQoL with the intervention. Qualitative studies will describe facilitators’ experiences of delivery of the intervention. A cost-effectiveness analysis (CEA) of the REACH-HF intervention in participants with HFpEF will estimate incremental cost per quality-adjusted life year at 12 months. The CEA will be conducted from a UK NHS and Personal Social Services perspective and a wider societal perspective. The adequacy of trial recruitment in an initial 6-month internal pilot period will also be checked.
Ethics and dissemination The study is approved by the West of Scotland Research Ethics Committee (ref 21/WS/0085). Results will be disseminated via peer-reviewed journal publication and conference presentations to researchers, service users and policymakers.
Trial registration number ISRCTN47894539.
- Self Care
- REHABILITATION MEDICINE
- Heart failure
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study compares an established rehabilitation programme with usual care for individuals with heart failure and preserved left ventricular ejection fraction and their caregivers.
Evaluation of a home-based model of intervention delivery that can improve access to rehabilitation services.
Due to the nature of the intervention, blinding of trial participants and clinicians to group allocation was not possible. Outcome assessment and data analysis were blinded.
Introduction
Heart failure (HF) is common, often leads to impaired physical function and reduced health-related quality of life (HRQoL) and increases morbidity, mortality and healthcare costs.1–5 At least half of people with HF have preserved ejection fraction (HFpEF).3 6 In contrast to HF with reduced ejection (HFrEF), for which there are several guideline-recommended pharmacological and non-pharmacological therapies that improve life expectancy and HRQoL, there are few for HFpEF, including sodium-glucose co-transporter 2 inhibitors.7 A recent meta-analysis of seven randomised controlled trials (RCTs) involving 346 participants with HFpEF shows that participation in exercise training may improve exercise capacity and HRQoL.8 Given the finite nature of this evidence base, larger multicentre trials with longer-term follow-up are still needed to confirm these potential benefits of exercise-based rehabilitation for HFpEF.
The Rehabilitation EnAblement in CHronic HF (REACH-HF) intervention is a comprehensive exercise-based rehabilitation and self-management programme informed by evidence, theory and service user perspectives designed for people with HF and their caregivers.9 As a home-based intervention, REACH-HF offers an alternative to traditional centre-based programmes and can improve access and uptake of rehabilitation.10 A multicentre RCT showed the REACH-HF programme was clinically effective and cost-effective for people with HFrEF.11 12
Additionally, a single centre pilot RCT in 50 participants with HFpEF allocated to receive REACH-HF or usual care alone demonstrated favourable trends, including improvements in disease-specific HRQoL (between group difference in Minnesota Living with Heart (MLwHF) Questionnaire total score (−11.5, 95% CI: −22.8 to 0.3 at 6 months follow-up) and cost-effectiveness.13 The pilot study supported the feasibility and acceptability of the REACH-HF intervention for participants with HFpEF and the RCT design.
Accordingly, the REACH-HFpEF trial was designed to investigate the clinical effectiveness and cost-effectiveness of a home, exercise-based rehabilitation programme for patients with HFpEF.
Aims and objectives
We aim to assess the clinical and cost-effectiveness of REACH-HF plus usual care (intervention) versus usual care alone (control) in participants with HFpEF and their caregivers.
The primary objective is to compare disease-specific HRQoL at 12-month follow-up between participants with HFpEF in the intervention and control groups.
Secondary objectives:
To check the adequacy of trial recruitment in an initial 6-month internal pilot study.
To compare the following secondary outcomes between participants with HFpEF in the intervention and control groups at 4-month and 12-month follow-up: exercise capacity, psychological well-being, level of physical activity, generic HRQoL, disease-specific HRQoL, self-management activities, frailty, prognostic biomarker, clinical events (death and hospital admission) and serious adverse events.
To estimate the cost-effectiveness of REACH-HF, compared with usual care alone, in participants with HFpEF as incremental cost per quality-adjusted life year (QALY) at 12 months post-randomisation.
To explore the moderators and mediators of change in the primary outcome of participants with HFpEF in the intervention group.
To qualitatively explore REACH-HF facilitators’ experiences of the delivery of the intervention.
To compare psychological well-being, HRQoL, self-care activities and burden between caregivers in the intervention and control groups at 4-month and 12-month follow-up.
To assess the fidelity of delivery of the REACH-HF intervention (to inform further future refinement/implementation in the UK NHS if the intervention is effective).
Methods and analysis
This protocol is reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials 2013 guidance.14
Design
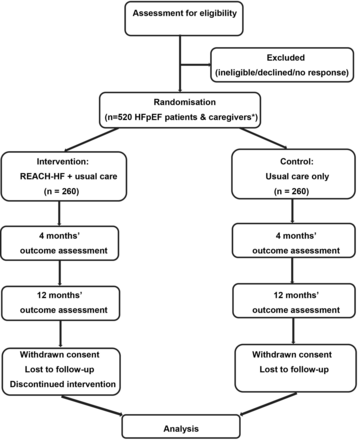
REACH-HFpEF is a multicentre parallel two group superiority RCT with nested process and health economic evaluations and an internal pilot phase. Given the complex nature of the REACH-HF intervention, it is not possible to blind participants or those involved in the provision of care beyond the point of randomisation. Researchers collecting outcome data and the statistician undertaking the data analysis will be blinded to treatment allocation to minimise potential bias. The RCT was registered on 15 December 2021 (ISRCTN47894539). An illustration of the study design is shown in figure 1.
Illustration of study flow. *Dependent on number of caregivers recruited.
Setting and recruitment
The study plans to recruit a total of 20 sites across England, Northern Ireland, Scotland and Wales. Patients are being recruited from both primary and secondary care pathways including HF registers and outpatient clinics. Follow-up procedures will usually be conducted on NHS premises. Conduct of the study will be led by a local principal investigator, supported by a research nurse or fellow and/or research assistant at each site, all of whom are trained in Good Clinical Practice (GCP) and in the requirements of the study protocol.
We have experienced a slower rate of trial recruitment of 0.8 patient/site/month compared with our predicted rate of 1.5 patients/site/month. As a result, we have implemented a number of strategies: (1) negotiated with our trial funder (NIHR) a 9-month extension to our recruitment closure date; (2) regular communication with our sites including quarterly trials newsletter, a weekly email to all sites of recruitment figures, and termly principal investigator/trial site team web meetings to discuss progress; and (3) introduction of a financial incentive to sites based on successful patient recruitment.
Study population
The study population includes eligible patients and caregivers. Participating patients will be aged 18 years or older and have a confirmed diagnosis of symptomatic HF with left ventricular ejection fraction ≥45% within the last 3 years prior to randomisation, confirmed by echocardiography or MRI. Patients who have undertaken cardiac rehabilitation within the last 12 months and those who have any contraindications to exercise training will be excluded. Inclusion and exclusion criteria are detailed in figure 2.
Patient with HFpEF inclusion and exclusion criteria. BMP, body mass index; BNP, B-type natriuretic peptide; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; HF, heart failure; NYHA, New York Heart Association.
Participants may choose to withdraw at any time and are given the option to fully withdraw from the study, or they can withdraw from the intervention and/or site visits but continue to complete the patient-reported outcome questionnaires only, especially the primary outcome of the MLWHF questionnaire. Data will be collected up to the point of withdrawal and used for analysis. If a participant deviates from the intervention protocol, they will be followed up as intention to treat. Participating caregivers will be aged 18 years or older and provide unpaid support to patients. Participant and carer consent forms are available to view as online supplemental files 1 and 2.
Supplemental material
Supplemental material
Randomisation
Participants will be randomly allocated in a 1:1 ratio to either intervention or control group. Randomisation will be stratified by investigator site and minimised on investigator site, sex and left ventricular ejection fraction (45–55% vs >55%). Randomisation will be achieved by using a secure web-based system. The research team will enter the participant identifier and the system will verify eligibility using data contained in the eCRF (electronic case record form).
Intervention
REACH-HF is a home-based CR programme providing self-care support to the patient and their caregiver.9 11 12 It was developed in cooperation with people living with HF and their caregivers, as well as service providers using an established rigorous intervention development framework9 to incorporate existing evidence, clinical guidance on HF self-care, behaviour change theory and key stakeholder perspectives. Table 1 provides an intervention description according to the Template for Intervention Description and Replication checklist.15
Details of the exercise component of the intervention are provided in table 2.
Summary of the REACH-HF intervention description according to the Template for Intervention Description and Replication15
REACH-HF intervention—exercise prescription for chair and walking programme
Usual care
Intervention and control patients will receive usual medical management as per clinical practice guidelines3 5 for treatment of participants with HFpEF. This includes the screening for both cardiovascular and non-cardiovascular comorbidities such as hypertension, diabetes mellitus, ischaemic heart disease and atrial fibrillation, which should be treated with safe and effective interventions that exist to improve symptoms, well-being and prognosis. Diuretics are recommended in those who are congested to alleviate symptoms. As part of usual care, all patients in the trial will be provided with the British Heart Foundation ‘Living with heart failure’ booklet.16 At the 4-month and 12-month follow-up, we will record any cotherapies received as part of usual care.
Outcome measures
All primary and secondary outcomes will be collected at baseline (prerandomisation) and 4-month and 12-month postrandomisation. At the time of follow-up, patients will be asked if they have had any adverse events. The principal investigators (PIs) will be required to report serious adverse events within 24 hours of becoming aware of the event to the pharmacovigilance office. Any serious adverse events occurring during the trial will be recorded and reported to the Ethics Committee and the Data Monitoring Committee.
Primary outcome
Patient disease-specific HRQoL data will be collected at 12 months postrandomisation through the MLwHF Questionnaire. This validated questionnaire consists of 21 items to assess the impact of living with HF on the key physical, emotional, social and mental dimensions of quality of life.17 It provides scores for two dimensions, physical and emotional, and a total score.
Secondary outcomes
Patients:
Exercise capacity (incremental shuttle walk test).18
Physical activity levels (accelerometry over a 9-day period, measured using the GENEActiv Original accelerometer).19
Psychological well-being measured using the Hospital Anxiety and Depression Scale questionnaire20
Generic health-related quality of life using EuroQol EQ-5D-5L questionnaire21
Generic health-related quality of life Short-Form-12 (SF-12)22
Kansas City Cardiomyopathy Questionnaire23
Frailty using the Clinical Frailty Scale24
Self-care of HF Index questionnaire25
Self-efficacy for key behaviours questionnaire.11
Biomarker of cardiac wall stress NT-proBNP level.
Clinical events assessed by deaths and hospital admissions (with HF-relatedness determined by an independent adjudication panel).
Caregivers:
Caregiver burden for HF Questionnaire.26
Caregiver contribution to Self-care of HF Index questionnaire.27
Family caregiver Quality of Life Scale questionnaire.28
Generic health-related quality of life using EQ-5D-5L.21
Psychological well-being using the HADS questionnaire.24
Summary of the study schedule is detailed in figure 3.
Summary of the study schedule. ISWT, Incremental Shuttle Walk Test; MLWHFQ, Minnesota Living with Health Failure Questionnaire; KCCQ, Kansas City Cardiomyopathy Questionnaire; SF-12, Short Form 12; HADS, Hospital Anxiety and Depression Scale; SCHFI, Self-Care of Heart Failure Index; FAMQOL, Family Caregiver Quality of Life Scale; CBQ-HF, Caregiver Burden Questionnaire for Heart Failure; CC-SCHFI, Caregiver Contribution to Self-Care of Heart Failure Index.
Sample size
At the design stage, the trial sample size was calculated in accordance with the DELTA2 guidance.29 A total of 520 (260 per group) participants with HFpEF is required for 90% power at 5% significance to detect a mean difference on the MLwHF Questionnaire of 5 points,17 assuming a SD of 20 points,13 a within patient correlation of 0.59 between baseline and 6 month follow-up, and an attrition rate of 15%. A 5-point difference in MLwHFQ score represents a minimum clinically important difference. Data from the REACH-HFpEF pilot trial13 indicate that the correlation between baseline and 6 months will be at least 0.59 (estimated correlation 0.73, 95% CI: 0.59 to 0.83).
Prior to the final analysis, in January 2025, the trial sample size was reassessed. A recent publication30 examining the responsiveness and minimal clinically important difference (MCID) of the MLwHF questionnaire suggested that a 16.6-point improvement represents a favourable outcome for patients. Based on a blinded access to trial data, specifically the overall distribution of changes in MLwHF scores, it was calculated that a mean between-group difference of 6.7 points in score at 12 months would equate to 50% more patients achieving a favourable outcome. Taking this as a MCID between groups, combined with the current baseline-adjusted residual SD in 12-month MLwHF scores of 17.8 points, and the current 12-month retention rate of 81%, the required sample size for 90% power at 5% significance was calculated to be 372. The rationale and basis of this updated sample size calculation were reviewed and approved by the Trial Steering Committee (TSC), Data Monitoring Committee (DMC) and trial patient & public involvement (PPI) group.
Trial data collection
All required study data will be captured in a set of purpose-built eCRFs. Access to the eCRFs will be restricted, via a trial-specific web portal, and only authorised personnel will be able to enter data. The site principal investigator or their designee(s) will be responsible for all entries into the eCRF and will confirm that the data are accurate, complete and verifiable. Data will be stored in a Microsoft SQL Server database at the University of Glasgow Clinical Trials Unit, which has an ISO 9001 quality management system and ISO 27001 for information security.
Participants will be able to complete their questionnaires on a paper CRF (that will then be entered into the eCRF by the local research team) or to complete them electronically. Where completed electronically, data will be entered directly into a participant-facing version of the eCRF. As the eCRF will be adapted for self-completion, consent will be sought to use the participant contact details provided for recontact to verify responses as needed. Participants who consent to long-term follow-up of their outcomes using routine data, NHS/Community Health Index numbers will be collected to facilitate the potential collection of data in the future.
Regular data management/cleaning will be undertaken to assess data quality. Quality assurance checks will be performed to monitor the level of missing data and the timeliness of data entry and check for inconsistent data.
Process evaluation
The process evaluation will assess the following research questions:
Was the intervention delivered as intended?
What adaptations were made/required in the intervention and do these impact outcomes?
Was the intervention used as intended?
What mechanisms explain any observed impact on patients’ HRQoL and other patient and caregiver outcomes?
What are the perspectives of patients, caregivers and service providers on the experience of being involved in REACH-HF?
What factors are associated with variation in intervention effectiveness among intervention recipients?
What adaptations were made within the service and did these impact fidelity and outcomes?
The process evaluation will use mixed methods at multiple case levels (patient, facilitator and centre) to test the programme theory in the population with HFpEF, identifying which components and configurations are best suited to meet their needs.31 32 The process evaluation will identify refinements of the programme theory, to optimise implementation and ensure that the essential ingredients of future interventions are better identified, interrogated and tested.33 As the analysis progresses, the implementation strategy will be revisited, focusing on potential outcomes such as Non-adoption, Abandonment, Scale-up, Spread, Sustainability Framework.34 This will maximise the clinical application of our research findings and enhance the capacity of staff working with participants with HFpEF to implement the intervention.
The participants in this process evaluation will comprise a subsample of patients, caregivers and REACH-HF facilitators taking part in the REACH-HFpEF trial. To answer the research questions, this mixed-method process evaluation will use trial primary and secondary outcomes and collect additional qualitative data (eg, intervention session recordings and interviews). The process evaluation will use multimodal longitudinal data.35 36
Process evaluation 1: participant and caregiver experience
15–20 patients (and 10 caregivers of these same patients) will be purposively selected and invited to take part in semistructured interviews. Patients will be chosen to represent, for example, diversity in terms of site/facilitator, sex, ethnicity, presence of a caregiver and baseline MLwHF Questionnaire total score.
The research team will interview each of these patients/caregivers at 4 months after the baseline visit (ie, immediately after intervention delivery is complete) and 12 months after the baseline visit. This will allow capture of patient and caregiver narratives over time, in relation to both intervention receipt and the longer-term impact/maintenance of self-care following the intervention. We will audio or video record these interviews, which may be conducted in person (if possible) or remotely (if not). Recording will use encrypted recording methods (either via password-protected online meeting software or an encrypted voice recorder). Written consent will be obtained prior to face-to-face interviews.
Topic guides for the interviews have been codeveloped with the patient and public involvement (PPI) advisory group. Interviews are designed to last between 30 min and 60 min. The researcher will endeavour to interview the patients without the caregiver present, where possible, and be mindful of the patient’s symptoms, such as fatigue or breathlessness, which may make an interview burdensome for the participant. The two interviews (and potentially selected segments of the intervention session recordings which represent good practice) will be transcribed verbatim. Thus, for each patient, their qualitative dataset is likely to comprise: two face-to-face meetings with their facilitator, five telephone meetings with their facilitator and two interviews with the process evaluation team.
Process evaluation 2: REACH-HF facilitator’s experience
In addition, 15 REACH-HF facilitators will be invited to take part in the process evaluation.
The process evaluation team will send an email to the participating facilitators with a brief questionnaire about their clinical background. This short questionnaire will either be completed in an electronic Word document and returned via email, or by following a link in the email to an electronic questionnaire (eg, using the electronic questionnaire platform Qualtrics). The process evaluation team will endeavour to sample REACH-HF facilitators to represent diversity in, for example, site, background training (eg, physiotherapy and nursing) and years of experience in delivery of cardiac rehabilitation (gathered using the clinical background questionnaire, see above). A topic guide will inform the interview, premised on the existing literature and gaps in current knowledge about intervention delivery. These interviews will be conducted either in person or remotely via telephone/web-call.
Verbatim interview transcripts will be organised and coded using MAXQDA. A framework analysis will be conducted, and sections of data relating to the aims of this research will be assigned a code that summarises the content either descriptively or interpretively. Codes with common features will be grouped together in themes, before finally being assigned to overarching themes. Where possible, data about self-reported behaviour from the interviews will be compared with observed behaviour evident in the intervention session recordings. A second qualitative researcher from the team will conduct independent analysis of a subset of the data. The researchers’ reflexive memo notes will enhance the integrity of the analysis.
The analysis will characterise patients’ and caregivers observed and self-reported responses to the intervention and link these responses to engagement with the intervention and perceived benefit, identifying interpersonal processes that shape the effectiveness or ineffectiveness of the intervention. At 4 months, patients’ and caregivers’ engagement with, response to and use of the REACH-HF manual will be characterised and differences between patients noted. At 12 months, overall use and benefit and maintenance of self-care behaviours and coping skills will be characterised and linked to individual differences in 4-month responses. Analysis will explore both patients’ and caregivers’ experiences of participation in the intervention and explicitly examine any potential impact of caregiver presence on patient adherence to the REACH-HF intervention.
Process evaluation 3: fidelity of intervention delivery
Facilitator-patient interactions (face-to-face and phone) for up to 60 patients will be audio-recorded (approximately 5–6 interactions taking 4–5 hours per patient). Recordings will be assessed using a previously developed and tested fidelity assessment checklist.8 The 12-item checklist uses a 0–5 rating scale based on the Dreyfus scale for assessing clinical competence.37 It focuses on assessing the quality of delivery of key delivery processes, such as the use of a patient-centred communication style, making a plan of action and encouraging self-monitoring of progress (particularly with the exercise programme). Intervention delivery fidelity data will be presented descriptively (mean scores with SD or 95% CIs) and broken down by site and by facilitator (as well as the calculation of overall delivery fidelity scores) for each checklist item. This will clarify how well intervention components were delivered and may identify ways to optimise delivery for future implementation. It will also allow researchers to describe variability in fidelity of delivery across patients and facilitators.
In addition, segments of the recordings that represent clear examples of good practice associated with each component of delivery (each item on the checklist) will be identified by noting the start/end timestamps of the segment within the audio file. These segments will be transcribed and collated for informing future REACH-HF training. Any information that might be used to identify the patient or the facilitator within the transcript will be redacted.
We will report descriptive statistics to summarise the fidelity of intervention delivery for each checklist item and will (descriptively and anonymously) examine variations between sites. Synthesis of the analysis of the intervention delivery fidelity and the interview data will enable a qualitative evaluation of potential pathways and barriers to improvement, which will pay attention to discrepancies between expected and observed outcomes, to understand how context influences outcomes and to provide insights to aid future implementation.
Process evaluation 4: facilitator checklist and log
REACH-HF facilitators will be asked to complete a brief self-rated fidelity checklist after each session they deliver. This comprises questions about the same 12 delivery fidelity components described above and allows the facilitators to rate the occurrences of each feature (absence, minimal, some, sufficient, good, very good and excellent). An independent observer rating is resource-intensive, while self-rated assessment may provide a pragmatic, real-world alternative to monitor delivery quality. The validity of the self-rating method will be checked by examining the correlation with observer-rated intervention delivery fidelity. We will also explore in the qualitative interviews whether use of the checklist facilitates/encourages reflexive practice and, in doing so, the quality of implementation.
Additionally, facilitators will be asked to complete a facilitator contact log for each participant. This log is a one-page pro forma designed to capture time, expenditure and any other resources required for the implementation of REACH-HF, as well as any adaptations made to the intervention for individual patients. It will capture data for both assessment of the fidelity of REACH-HF delivery and economic analyses.
A detailed process evaluation analysis plan will be drafted prior to study data lock and agreed with the Trial Management Group (TMG) and TSC.
Economic evaluation
Economic analysis will be performed to establish the cost-effectiveness of REACH-HF plus usual care compared with usual care alone. Following on from the results of the economic evaluation pilot study,13 a within-trial cost-utility analysis will be conducted. Pilot study findings revealed differential resource distributions across primary, secondary and social care as well as impacts on informal carer time and costs. Bespoke data capture instruments have been developed to ensure capture of all relevant resource use from both an NHS/Personal Social Services (PSS) perspective, as well as a broader societal perspective. There is evidence of insensitivity of the EQ-5D-5L in patients with mild HF.36 38–41 A recent study comparing the EQ-5D-5L and short-form six-dimension (SF-6D) in elderly participants with HF recommends use of SF-6D in those with milder disease and economic outcomes.39 Therefore, we propose to use both the SF-6D (from SF-12) and the EQ-5D-5L. As recommended by NICE economic evaluation guidance, the base-case perspectives will be that of the UK NHS and PSS.42 Further, a broader societal perspective, accounting for resource use, productivity (employment) and personal cost impacts faced by patients and their carers will be considered in sensitivity analyses, along with a scenario analysis incorporating HRQoL values obtained from mapping MLwHF Questionnaire scores to EQ-5D utilities, using a validated mapping algorithm.43 44 The base case economic evaluation will estimate the incremental cost per QALY associated with the REACH-HF intervention, compared with usual care alone, and will be reported in line with updated reporting guidelines for economic evaluations.45 The wider societal perspective will incorporate resource use, productivity (employment) and personal costs. Missing resource use and outcome data will be handled using multiple imputation.46 If within-trial results reveal between-group differences in HRQoL, a decision analytic model will be developed to estimate the cost-effectiveness results over a lifetime horizon.
A detailed health economic analysis plan will be drafted prior to study data lock and agreed with the TMG and TSC.
Statistical analysis
Participation from screening to completion of the final follow-up assessment will be reported. Baseline patient characteristics and outcome scores will be summarised descriptively.
The primary statistical analysis for both primary and secondary outcomes will take an intention to treat approach (according to randomised allocation) based on complete data. For continuous outcome measures, mixed-effects regression will be used with a random effect of recruiting site and adjusting for baseline outcome score and minimisation variables. Additional clustering of outcomes due to therapist effects will be accounted for in sensitivity analyses.
A number of secondary analyses will be undertaken. Patterns and reasons for missing outcome data will be assessed, and sensitivity analyses will use appropriate imputation models to assess the impact of missing data. Potential subgroup treatment effects will be explored by adding treatment-by-subgroup interaction terms to analysis models. Potential subgroups assessed will include sex, study site and participant baseline NT-proBNP levels, ejection fraction and important markers of inequity, such as age, socioeconomic status and having a carer. Since the trial is powered to detect overall differences between the groups rather than interactions of this kind, these subgroup analyses will be regarded as exploratory. Before the start of recruitment, the TMG (with TSC approval) will be asked to define the minimum adherence to the REACH-HF intervention required to indicate compliance. Complier average causal effects analyses will be used to estimate the causal intervention effect in relation to each outcome.
Adherence will be defined using criteria adapted for the delivery processes proposed for the current study. These criteria will be developed with the TMG, building on the criteria used in the prior multicentre REACH-HF trial in people with HFrEF.11 Associations between physiological, cognitive and demographic factors and intervention adherence will be explored.
Estimated between-group differences will be presented using both absolute and relative measures, with associated 95% CIs, where appropriate. No correction of p values for multiplicity of testing will be undertaken. However, the analysis for the primary outcome will be performed before all other analyses, and the p values of all subsequent analyses will be interpreted in the context of multiple testing. No interim analyses are planned. Safety/adverse event outcomes will be reported descriptively by group.
A detailed statistical analysis plan will be drafted prior to study data lock and agreed with the TMG and TSC.
Substudies
Three prespecified substudies are being undertaken alongside the main REACH-HF trial.
Study within a trial (SWAT): the objective of the SWAT is to determine if an evidence-based enhanced participant information sheet impacts on recruitment and retention of caregivers to a multicentre host trial. Embedded in the main trial, the SWAT will be a cluster RCT design with allocation of the trial sites to either the enhanced host trial caregiver PIS (SWAT intervention group) or the standard host trial caregiver PIS (SWAT control group). The SWAT is led by University College Dublin and is registered with the ISRCTN trial registry (ISRCTN15757498) and the MRC SWAT Repository (https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,1218962,en.pdf). The SWAT protocol will be submitted for publication separately.
Optimisation of exercise fidelity in home-based cardiac rehabilitation study. This substudy aims to apply novel indicators of exercise fidelity (ie, quality of exercise in relation to the exercise prescribed) in the participants with HFpEF participating in the main trial. By identifying measurable indicators of exercise fidelity and associating them with patient outcomes, the substudy intends to identify ways to assess and tailor future home-based exercise interventions. Assessing the quality of the patients' exercises might also give them useful feedback about their progress and how they can get more benefit from the exercise component in future implementations of the REACH-HFpEF (or other home-based exercise interventions). This substudy is led by the University of Birmingham.
This substudy will seek a sample of up to 80 intervention group patient participants with a tracker watch and mobile phone and a brief questionnaire. These will be used to: (a) measure resting heart rate pre and post intervention (b) monitor heart rate during all their REACH-HFpEF exercise sessions and (c) video-record 1–2 exercise sessions to check for safety and accuracy of Quantitative
Mediation Analysis: the proposed statistical mediation sub-study will form an extension of the main trial process evaluation and aims to assess the association of the change of secondary outcomes as potential mediators of the REACH-HFpEF intervention primary outcome measure (MLwHF questionnaire). This substudy is led by the University of Exeter.
Data monitoring and quality assurance
Trial-specific work instructions will be developed in accordance with University of Glasgow Clinical Trial Unit procedures. Regular data management and cleaning will be undertaken to assess data quality. Quality assurance checks will be undertaken to monitor the level of missing data and the timeliness of data entry and check for illogical or inconsistent data. The research team will monitor data collection procedures, ensuring that study data entry procedures are followed. The sponsor has categorised this trial as low risk and will therefore not be routinely monitored. The trial may be subject to audit by the sponsor.
Trial management and independent committees
The Trial Operations Group (TOG) team members directly involved with the day-to-day running of the trial (co-chief investigators (CC/RST) and trial managers (EB/COH/AP/ET) and trial administrator) will meet on a 2-week basis to monitor and discuss the day-to-day management and all aspects of progress of the study. The TOG will have regular contact with trial sites by email and webinar meetings. The TMG, including the health economics, statistics, process evaluation teams, co-applicants and PPI representation, will meet on a termly basis to review the status of the study and trial progress.
The REACH-HFpEF TSC consists of independent members with clinical and trial methodological expertise and includes a patient and public involvement representative. The TSC will provide independent oversight of the conduct, timelines and funding of the trial with safety and ethics review by an independent DMC. The TSC and DMC will normally meet one to two times per year. Detailed descriptions of the remit and function of the committees are documented in specific charters held in the Trial Master File by Glasgow Clinical Trials Unit.
Patient and public involvement
A PPI group will be established for this trial: 12 participants with lived experience of HFpEF and their partners/carers. These patients are usually managed and monitored in general practice.12 We will advertise on the NIHR People in Research website to recruit these patients and their partners/carers to the PPI group. An induction webinar will be held to introduce the group to the study and to negotiate characteristics of the PPI role throughout the study, including training and support needs.
Additionally, PPI representatives were members of the TMG and TSC.
Ethics and dissemination
The study will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with ICH GCP, and in accordance with the Research Governance Framework for Health and Social Care, Second edition (2005). The study and all relevant study documents have been reviewed and approved by the West of Scotland Research Ethics Service (reference number 21/WS/0085). The study sponsor is The NHS Greater Glasgow and Clyde. Written informed consent will be obtained from all study participants by the PI or designee prior to enrolment in the trial. All protocol modifications are being communicated to Research Ethics Committee (REC), funder, sponsor, TSC and DMC.
Study results will be published in open access publications in high impact peer-reviewed journals, including an end of trial NIHR monograph, and will be presented at national and international conferences. The study will be featured at the stakeholder dissemination workshop (with patients, clinicians, commissioners, academics and key groups such as British Heart Foundation, British Association for Cardiovascular Prevention and Rehabilitation and Pumping Marvellous). Direct feedback will be given to trial participants, and information will be digitally publicised on the REACH-HF website and relevant profiles on social media platforms.
Trial status
The first participant with HFpEF was recruited in May 2022. The trial has opened 20 sites in England, Scotland and Wales (see appendix for listing) and as of 22nd May 2025 has recruited 382 participants with HFpEF and 94 caregivers.
Ethics statements
Patient consent for publication
Acknowledgments
The authors thank the study administrators, Kate Campbell and Lisa Hall, and all study participants and their caregivers. The authors also thank: Patient and Public Involvement group members (Dr Tracy Ibbotson [chair], Gary Curlewis, Rashmi Kumar, Robert Panton, Deborah Smith and Wade Tovey); Trial Steering Comimttee members (Prof Klaus Witte [chair], Prof Susan Dawkes, Tom Kennedy [PPI representative], Andrew Leatherland, Dr Ramon Luengo-Fernandez, Prof Helen Parsons and Prof Simon Williams); Data Monitoring Committee members (Prof John Norrie [chair], Prof Abdallah Al-Mohammad and Prof Ann Dorthe Zwisler); and Study site principal investigators: Dr Ify Mordi (Tayside Health Board), Drs Victor Chong & Anysley Cowie (NHS Ayrshire and Arran), Drs Matt Lee, Pierpaolo Pellicori & Karen Hogg & (NHS Greater Glasgow & Clyde), Dr Andrew D'Silva (Guy’s and St Thomas’ NHS Foundation Trust), Dr Rosita Zakeri (King’s College Hospital NHS Foundation Trust), Drs Yasath Samarage & Gerry Murphy (County Durham and Darlington NHS Foundation Trust), Dr Prathap Kanagala (Liverpool University Hospital NHS Foundation Trust), Dr Fozia Ahmed (Manchester University NHS Foundation Trust), Dr Matthew Dewhurst (North Tees and Hartlepool Hospital NHS Foundation Trust), Dr Justin Zaman (West Suffolk NHS Foundation Trust), Dr Piers Clifford (Buckinghamshire Healthcare NHS Trust), Dr Joe Martins (The Dudley Group NHS Foundation Trust), Dr John Walsh (Nottingham University Hospitals NHS Trust), Dr James Gamble (Oxford University Hospitals NHS Foundation Trust), Dr Andrew Ludman (Royal Devon and Exeter NHS Foundation Trust), Dr Chris Hayes (York and Scarborough Teaching Hospitals NHS Foundation Trust), Dr Joseph Mills (Wirral Community Health and Care NHS Foundation Trust), Prof Iain Squire (Leicestershire Partnership NHS Trust), Dr Philip Campbell (Aneurin Bevan University Health Board), Dr Will Watson (Cambridge University Hospital NHS Foundation Trust) and Dr Ameet Bakhai (Royal Free London NHS Foundation Trust)
References
Footnotes
RST and CCL are joint senior authors.
X @CNRGCam
Collaborators The REACH-HFpEF investigators.
Contributors RT, JGFC, AC, HMD, CD, PJD, JF, CG, NH-E, TI, KJ, AM, EM and CL developed the grant proposal for this trial. AP and RT prepared the draft protocol paper. The manuscript was revised based on comments from all authors (RT, EB, CO, EAT, AP, JCB, JGFC, AC, HMD, CD, PJD, KD, HF, JF, CG, NH-E, MH, TI, MJ, KJ, AM, EM, VS, IS, LT, SvB and CL). All authors read and approved the final manuscript. RST is responsible for the overall content as guarantor.
Funding This work was supported by the National Institute of Health Research, grant number: 130487.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.