Article Text
Abstract
Introduction Labour pain is an unavoidable feature of childbirth and is characterised by extreme intensity. Adequate pain management is thus essential not only from the aspect of physiological pain but also due to the adverse effects of pain on the psychological well-being of parturients. Many studies have shown the benefits of acupoint hot compress. However, to date, little is known about its use for alleviating labour pain. The purpose is to evaluate the effect of acupoint hot compress on relieving pain in primiparous women during the latent phase of the first stage of labour, as well as its effects on key maternal and neonatal outcomes.
Methods and analysis This prospective, multicentre, randomised controlled trial will be conducted across 18 institutions in China from January 2024 to August 2025. A total of 1100 primiparous women aged 20–34 years, with singleton pregnancies at 37–41 weeks of gestation, will be enrolled and randomly allocated to two groups using a central stratified block randomisation method. The controls will be treated only with obstetrical care, while those in the intervention group will receive the same obstetrical care as the control group, with the addition of acupoint hot compress therapy at 42±2°C for 4 hours, starting 1 hour after the onset of regular uterine contractions during the latent phase of labour. The primary outcome will be the pain intensity measured at 1, 3 and 5 hours after the onset of regular uterine contractions using a Visual Analog Scale.
Ethics and dissemination The study has been approved by the ethics committee of Women’s Hospital, School of Medicine, Zhejiang University (No. IRB-20230379-R). The results of the main trial will be submitted for publication in a peer-reviewed journal.
Trial registration number This trial is registered at Chinese Clinical Trial Registry, ChiCTR2300079244.
- COMPLEMENTARY MEDICINE
- Pregnant Women
- Puerperal Disorders
- PAIN MANAGEMENT
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study will employ a multicentre, prospective, randomised controlled design across 18 institutions.
Central stratified block randomisation with stratification by clinical centre will be implemented to minimise selection bias.
No blinding method will be used due to the lack of a feasible placebo for the hot compress intervention.
A standardised, non-individualised treatment protocol will be applied, potentially limiting personalisation of acupoint therapy.
Fixed-size hot compress devices may unintentionally stimulate adjacent acupoints, confounding intervention specificity.
Introduction
Pain is described as an unpleasant subjective sensation and represents one of the five vital signs.1 Labour pain is an unavoidable feature of childbirth and is characterised by extreme intensity.2 Adequate pain management is thus essential, not only for addressing physiological pain but also for mitigating the negative effects of pain on the psychological well-being of parturients.3 The presence of fear and anxiety may adversely affect the parturients’ health and may also influence the decision to request an elective caesarean section.4–6 Hence, the discovery of an effective method for the alleviation of labour pain is needed.
The treatments for relieving labour pain are classified primarily into pharmacological and non-pharmacological approaches.7 Medication is the most frequent form of pain relief used in mainstream medical practice.8 9 However, the possible negative consequences of the drugs on both the mother and fetus have led to interest in non-pharmacological treatments to reduce labour pain during childbirth.10 11
As noted in ACOG Committee Opinion No. 766, non-pharmacologic pain management techniques may be beneficial for women experiencing pain during the latent phase of labour.12 Several studies have indicated that non-pharmacological interventions have shown promising results in alleviating labour pain during the latent phase of labour. A systematic review of relaxation techniques for labour pain management has suggested that methods such as relaxation and music therapy could reduce pain and improve satisfaction with pain relief during the latent phase.13 Another research study has revealed that massage therapy can effectively alleviate labour pain during the latent phase.14
Acupoint hot compress, as a non-pharmacological intervention, is a non-invasive treatment based on the principles of Traditional Chinese Medicine (TCM). Many studies have shown the benefits of acupoint hot compress; notably, it is inexpensive, simple to use, safe and effective and can assist in reducing pain as well as preventing emotional disturbances and disorders.15–17 However, to date, little is known about its use for alleviating labour pain, particularly during the latent phase of the first stage of labour. We thus plan to conduct a randomised controlled clinical trial to investigate the efficacy of acupoint hot compress in the reduction of labour pain in primiparous women during the latent phase.
In Chinese clinical practice, pregnant women are typically hospitalised during the early stages of labour, often before the onset of regular uterine contractions, driven by cultural preferences and the healthcare system’s emphasis on close monitoring during childbirth.18 This ensures that participants in our trial will already be under medical supervision when interventions are initiated. The acupoint hot compress will be administered in hospital settings by trained obstetric professionals (obstetricians, midwives and nurses) who have completed standardised training in this technique. These professionals will closely monitor the participants to ensure that the intervention is administered safely and effectively.
Methods and analysis
Objective
This study aims to evaluate the effect of acupoint hot compress on pain relief in primiparous women during the latent phase of labour, as well as its impact on key maternal and neonatal outcomes, including labour duration, intrapartum and postpartum bleeding, maternal depression symptoms and neonatal Apgar scores.
Hypothesis
We hypothesise that using acupoint hot compress during the latent phase of labour will effectively alleviate labour pain, reduce labour duration, lower intrapartum and postpartum blood loss, ease maternal depression symptoms and improve neonatal Apgar scores.
Design of the trial
This study is a prospective, multicentre, open-label, randomised controlled trial. It will enrol 1100 primiparous women across 18 clinical centres in China. Participants will be allocated 1:1 to either the control (standard obstetric care) or intervention group (standard care plus acupoint hot compress). Participants may withdraw voluntarily at any time, with the reasons documented to assess potential bias. The trial may be terminated early if significant safety concerns (eg, severe intervention-related adverse events (AEs)) arise. The protocol follows the Standard Protocol Items: Recommendations for Interventional Trials guidelines19 (online supplemental material 1) and will be undertaken in accordance with the Consolidated Standards of Reporting Trials20 guidelines (figure 1).
Supplemental material
Flow chart of study procedures.
Recruitment of participants
Recruitment will take place from January 2024 to August 2025. Eligible primiparous women will be identified on hospital admission for labour, with obstetricians, midwives and nurses screening participants based on inclusion and exclusion criteria. After receiving a comprehensive explanation of the purpose, procedures, risks and benefits regarding the study, eligible women will provide written informed consent (online supplemental material 2). Consented participants will be enrolled and randomised into the control or intervention group using a central stratified block randomisation method. To ensure adequate enrolment, regular training sessions will be conducted for obstetricians, midwives and nurses at each centre to familiarise them with the study protocol and recruitment process. The study will be undertaken at 18 clinical centres in China: Women’s Hospital, School of Medicine, Zhejiang University; Gansu Provincial Maternal and Child Health Care Hospital; Hangzhou First People’s Hospital; Hangzhou Women’s Hospital; Yiwu Maternity and Children Hospital; Jiaxing Maternity and Child Health Care Hospital; The Second Affiliated Hospital of Wenzhou Medical University; Lishui Central Hospital; Wenzhou Central Hospital; Hangzhou Fuyang Women and Children Hospital; Hangzhou Linping District Maternity and Child Health Care Hospital; The Fourth Affiliated Hospital, Zhejiang University School of Medicine; Tonglu Maternal and Child Health Hospital; The First People’s Hospital of Jiashan; The First Affiliated Hospital of Ningbo University; The First People’s Hospital of Xiaoshan District; Tongxiang Maternal and Child Health Hospital; Huzhou Maternity and Child Health Care Hospital. Recruitment will not discriminate against any particular cultural, racial or socioeconomic group.
Supplemental material
Inclusion criteria
The inclusion criteria are: age ranging from 20 to 34 years; gestational age ranging from 37 to 41 weeks; primiparous parturient women with planned vaginal delivery; singleton pregnancy; meet the diagnostic criteria of the latent phase of the first stage of labour;21 ability to communicate well with the researcher and comply with the test requirements; and provided written informed consent.
Exclusion criteria
Prior uterine surgery or uterine abnormalities.
Fetal anomalies, chromosomal abnormalities or stillbirth.
Prenatal diseases, including infectious, central nervous system, urogenital diseases and internal and surgery-related diseases, as well as the use of long-term medication.
A history of psychiatric or neuropsychological disorders, as well as impaired verbal communication.
The presence of severe diabetes, skin diseases, sensory disorders, acute inflammation, high fever and allergies to the material used in the study product.
Patients with incomplete information.
Withdrawal criteria
AEs related to the intervention (eg, skin burns, severe allergic reactions) occur.
Participants fail to comply with the study protocol.
Participants will have the option to voluntarily withdraw at any time and for any reason.
Dropout criteria
Change from vaginal delivery to caesarean section.
Non-completion of the intervention.
The principal researcher will have the authority to conclude the study at any time when the benefits are considered to outweigh the risks.
Poor treatment compliance by the subject.
Calculation of sample size
The sample size calculation will be based on the primary outcome of pain intensity during the latent phase of labour, rated on a Visual Analog Scale (VAS). The pilot study was conducted with 50 participants (25 in the control group and 25 in the intervention group). The difference between the means of the two groups was found to be 0.61 with an SD of 1.08. With 90% power, a two-sided threshold of 0.05 and a 20% dropout rate, the size of the sample was found to be 1100, with 550 participants in each group. The pilot study sample was representative of the main study population in terms of demographic characteristics and clinical setting, ensuring the generalisability of the findings.
Randomisation and blinding
The eligible subjects will be randomly allocated to the control and intervention groups in a 1:1 ratio using a central stratified block randomisation method, which will be generated by an independent statistician using conventional statistical computer software (SPSS V.25.0). No blinding method will be used due to the lack of a feasible placebo for the hot compress intervention.
Interventions
Control group
The participants will receive only obstetrical care, including monitoring of maternal vital signs, fetal position and heart rate, uterine contractions, vaginal bleeding and labour progress, as well as conventional pain management (breathing techniques and massage) and psychological and social support.22
Intervention group
Participants in the intervention group will receive the same obstetrical care as the control group (detailed in Control group), including the conventional pain management. Additionally, they will receive acupoint hot compress therapy at 42±2°C for 4 hours, starting 1 hour after the onset of regular uterine contractions during the latent phase of labour. Specifically, regular contractions refer to uterine contractions that occur at a consistent frequency (every 5–6 min) and duration (lasting at least 30 s).22 To ensure accuracy, we adopt a combination of clinical assessments to measure and confirm the presence of regular contractions. These assessments primarily include: periodic palpation by midwives or obstetricians to evaluate the regularity, intensity and pattern of the contractions. Furthermore, cervical examinations are conducted to further validate the contractions by evaluating cervical dilation and effacement.
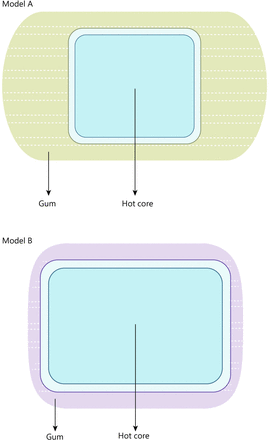
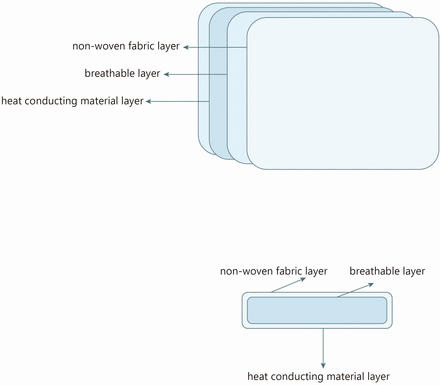
For the intervention, this will be applied using a Hu-Chao-Nuan-Gong-Bao, a licensed Class II item of medical equipment (licence No. 20192090292) manufactured by Jiangxi Shenghe Industrial Development (Nanchang, China). Acupoint hot compress includes two sizes (figure 2), model A (measuring 16×9 cm) and model B (measuring 13×10 cm). These comprise four hot cores, each measuring 8×7 cm (model A), as well as one hot core measuring 11.5×8 cm (model B). Agents such as inorganic salts and activated carbon can be placed within the hot cores in specific proportions in a sealed inner bag. The temperature can be maintained at a constant level due to the specific rates of oxygen and water vapour transmission within the breathable layer (figure 3).
Two styles of acupoint hot compress.
Structure diagram of hot core.
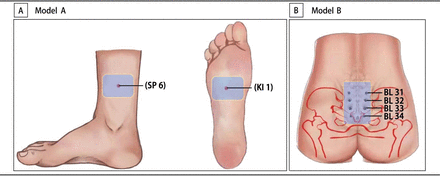
The selection of acupoints will be determined according to our hospital’s clinical experience and in consultation with 10 experts. The Nomenclature and Location of Acupuncture Points (National Standard of the People’s Republic of China, 2006 (GB/T 12346-2006)) describes the acupoint locations.23 As shown in figure 4, two of the hot cores shown in model A will be applied separately on Bilateral Yongquan (KI1) (figure 4A) 1 hour after the onset of regular uterine contractions, while two of the other hot cores in model A will be applied separately on Bilateral Sanyinjiao (SP6) (figure 4A) and one hot core from model B will be applied on Baliao (BL31–34) (figure 4B).
Locations of acupoint hot compress. (A): Model A will be applied on Sanyinjiao (SP6) and Yongquan (KI1). (B): Model B will be applied on Baliao (BL31-34).
Primary outcome
Labour pain
Pain during the latent phase of labour will be assessed using a VAS in which, on a scale of 0–10, where ‘0’ represents ‘no discomfort’ and ‘10’ indicates ‘terrible pain’. Pain intensity will be assessed using the VAS at 1, 3 and 5 hours following the onset of regular uterine contractions. The VAS is a widely validated and reliable tool for pain assessment, demonstrating high sensitivity in detecting pain intensity variations.24 To reduce potential confounding effects, participants will be allocated using a central stratified block randomisation method to ensure balanced group allocation. Moreover, all clinical centres will follow the same standardised protocols for labour monitoring and pain assessment to minimise variability.
Secondary outcomes
Labour duration
The labour duration will be measured from the start of labour until birth. The durations of all labour stages (first, second and third), as well as the overall duration, will be recorded.
Blood loss
Blood loss estimation after the delivery and at 2 hours postpartum will be recorded. The method of measuring blood loss is the quantitative measurements of the blood volume and weight.25 The quantification of the blood loss is as follows: a calibrated under-buttocks drape will collect all fluids immediately after delivery. The total volume of collected fluid will be recorded, and preplacental fluids (eg, amniotic fluid, urine) will be subtracted to isolate blood loss. Blood-soaked materials (eg, gauze, pads) will be weighed, and blood volume calculated as: blood volume (mL)=wet weight (g)−dry weight (g). Cumulative blood loss will be determined by combining the volumes from the drapes and weighed materials.
Symptoms of depression
The symptoms of depression may cover physical, emotional and cognitive abnormalities. Depressed mood and a loss of interest or pleasure are the primary symptoms of depression. The Edinburgh Postnatal Depression Scale (EPDS) will be administered within 48 hours postpartum to identify depression symptoms. The EPDS is a well-validated instrument widely used to assess depression and anxiety symptoms in perinatal populations.26 27 Participants will complete the EPDS questionnaire independently, as it is a self-reported tool. The completed questionnaires will be collected and recorded by trained researchers.
Apgar scores
The Apgar score is used to assess the five physical signs of the newborn, including pulse, respiration, appearance, muscle tone and reflex irritability. The Apgar scores will be documented at 1 and 5 min after delivery. The Apgar score serves as a predictive indicator for neonatal mortality, morbidity and long-term neurodevelopmental outcomes.28 29
Safety
The medical device to be used is a hot compress, which has been authorised by the Medical Products Administration under licence number 20192090292.
The pilot study did not find any AEs. The potential AEs will be described to the participants in this study, and people with sensory abnormalities will be excluded.
Data collection and management
Standardised training for conducting the study and the involved procedures will be provided to all researchers. All data will be collected using standardised case report forms and entered into a secure electronic database with a double data entry system to minimise errors. Regular audits conducted by the research team will ensure data accuracy and completeness. Access to the database will be restricted to authorised personnel only, and all data will be anonymised to protect participant confidentiality. Incomplete or missing data will be documented and addressed through follow-up with the participating clinical centres.
Statistical methods
Independent analysts will conduct the statistical analysis using SPSS V.25.0. All analyses will be undertaken using the intention-to-treat approach. No interim analyses will be conducted. For continuous data, t-tests, covariance analysis or Wilcoxon-Mann-Whitney tests will be used for analysis, while for categorical data, χ2 tests or Fisher’s exact tests will be used. In addition, a logistic regression analysis will be undertaken to determine associations between the independent variables.
Ethics and dissemination
The study has been approved by the ethics committee of Women’s Hospital, School of Medicine, Zhejiang University (No. IRB-20230379-R). This report is based on protocol version 2.1, dated 16 May 2024. Any modifications to the protocol will be submitted for ethics approval and updated in the ChiCTR. All participants will receive a comprehensive explanation of the purpose, procedures and potential risks associated with the trial and will be required to provide informed consent before taking part. The data obtained in the study will be made available by the corresponding author on reasonable request. The results will be published in international scientific journals.
Discussion
In our study, we aim to address labour pain relief, a critical aspect of maternity care that aligns with the WHO Sustainable Development Goals prioritising maternal and newborn healthcare.30 These goals emphasise reducing pregnancy-related complications, a leading cause of global maternal mortality.31 Effective interventions across all stages of maternity care are essential to achieve these objectives. Our focus on labour pain management during the latent phase reflects a key priority in maternal health, given its significant impact on childbirth outcomes.32 33
Currently used methods for labour pain relief include pharmacological approaches, such as epidural analgesia, narcotics and intravenous opioid administration,34 35 as well as non-pharmacological interventions, including acupuncture, hot compress, massage and music therapy.36–38 Most pharmacological methods are expensive and can have negative effects on both the mother and newborn.39 This has led to increased exploration of non-pharmacological techniques, which may enhance the delivery experience.
The hot compress is an economical, safe and non-invasive non-pharmacological method for pain management.40 According to the gate control theory, the hot compress can stimulate endorphin release, thereby reducing pain perception by inhibiting the transmission of pain signals to the brain.41 Additionally, the hot compress dilates blood vessels, enhancing local blood circulation and further contributing to pain relief.42
Building on these physiological mechanisms, acupoint hot compress integrates the benefits of heat therapy with TCM by targeting specific acupoints. Compared with other therapies, acupoint hot compress is particularly well received by patients and their families due to its non-invasive nature, low cost and psychological comfort.16 The combination of physiological and psychological benefits makes acupoint hot compress a promising intervention for labour pain management.
It should be noted that pain intensity and tolerance during the latent phase demonstrate significant interindividual variability. While some women may experience mild discomfort requiring no intervention, others may perceive the pain as intolerable and actively seek relief.43 Given this heterogeneity in pain perception, we emphasise that decisions regarding pain relief administration should be made on a case-by-case basis, considering each woman’s preferences, pain tolerance and the potential risks and benefits of available interventions. To ensure both methodological rigour and participant safety, our study will exclude individuals with psychiatric disorders or sensory abnormalities. This exclusion criterion is based on two considerations: First, psychiatric conditions may confound self-reported pain assessments44 45 and affect the validity of EPDS scores for depression evaluation. Second, sensory abnormalities increase the potential risk of thermal injury from the hot compress intervention.
There remain some limitations in this protocol. First, since there is no suitable placebo for the hot compress intervention, the group randomisation is not concealed from the subjects. This lack of blinding may introduce performance bias, since subjects’ awareness of group allocation may bias pain perception and reporting, potentially overestimating the efficacy of the intervention. Second, while it is widely recognised that TCM should be tailored based on syndrome differentiation, our study adopts a uniform treatment protocol for all participants. This approach may limit the generalisability of the findings, as the lack of individualised treatment could reduce the effectiveness of intervention for certain participants, leading to variability in outcomes. Third, the fixed sizes of the hot compress devices may not fit all participants’ body shapes and acupoint locations. This could cause either inadequate stimulation of the target acupoints or unwanted stimulation of nearby ones, possibly introducing bias in measurements.
In summary, the findings of our study will have important practical implications for the management of labour pain. First, acupoint hot compress represents a cost-effective alternative to pharmacological methods for pain management during labour, which can be particularly beneficial in resource-limited settings. Second, the technique is safe and does not require specialised training, making it feasible for widespread use among healthcare providers. Third, the use of acupoint hot compress may improve the experience of the latent phase for primiparous women, leading to psychological benefits and better perinatal outcomes. Furthermore, this study only includes short-term observation indicators but lacks long-term outcomes, such as postpartum recovery and infant development, which may limit the evaluation of the effects. Future studies should incorporate long-term follow-up to assess the sustained benefits and potential risks of acupoint hot compress for labour pain management.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to thank all the participants.
References
Footnotes
XL and YP contributed equally.
Contributors FQ conceived and formulated the trial, reviewed and revised the manuscript. XL and YP wrote the manuscript. NL, TZ, YL, YZ, FW and DX contributed to the revision of the manuscript. All authors read and approved the final manuscript. FQ is the guarantor.
Funding This study was supported by the Health High-Level Talent Training Project (Innovative Talents), the Health Commission of Zhejiang Province (File (2021) 40). The funding bodies had no role in the study design or writing of this manuscript.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.