Article Text
Abstract
Objective To assess the effectiveness of random capillary blood glucose as a diagnostic tool for type 2 diabetes and determine optimal cut-off values for adults in Bangladesh.
Design Cross-sectional diagnostic accuracy study.
Setting 16 diabetes centres were selected randomly from all eight administrative divisions of Bangladesh.
Participants A total of 3200 adults aged 18 years and older were recruited using systematic random sampling between May and September 2022.
Primary and secondary outcome measures The primary outcome was the diagnostic accuracy of random capillary blood glucose compared to fasting plasma glucose, 2-hour plasma glucose after a 75-gram glucose load and glycated haemoglobin. Secondary outcomes included sensitivity, specificity, area under the curve and agreement with the other diagnostic tests.
Results Random capillary blood glucose showed a strong positive correlation and high concordance with fasting plasma glucose, 2-hour plasma glucose and glycated haemoglobin. A cut-off value of ≥8.7 mmol/L demonstrated improved diagnostic performance compared with the currently used cut-off of ≥11.1 mmol/L. This new threshold yielded higher sensitivity, specificity, area under the curve and agreement with other standard diagnostic tests. Notably, hyperglycaemic symptoms were not required for diagnosis. The number needed to screen to identify one case of type 2 diabetes using the ≥8.7 mmol/L cut-off was 2.74, lower than that for fasting plasma glucose (2.86) and random capillary blood glucose ≥11.1 mmol/L (4.68).
Conclusions Random capillary blood glucose may be an effective and affordable diagnostic tool for type 2 diabetes in resource-limited settings. The proposed cut-off of ≥8.7 mmol/L offers improved diagnostic accuracy and reflects the population’s glucose distribution pattern.
- Type 2 diabetes
- random capillary blood glucose
- diagnostic accuracy
- Bangladesh
- screening
- oral glucose tolerance test
- primary care
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request from BB, the study principal investigator.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Type 2 diabetes
- random capillary blood glucose
- diagnostic accuracy
- Bangladesh
- screening
- oral glucose tolerance test
- primary care
STRENGTHS AND LIMITATIONS OF THIS STUDY
A large, systematically sampled population was included from all eight administrative divisions of Bangladesh.
All biochemical measurements were conducted using quality-controlled, centralised laboratory procedures.
The use of the oral glucose tolerance test as a reference standard enhances diagnostic comparison.
The study’s focus on a specific population may limit its generalisability to other regions or ethnic groups.
This study did not assess individual metabolic differences, variations in food intake before the test, different time points or the inherent variability of random capillary blood glucose measurements, which limits the explanation of glycaemic variance.
Introduction
Type 2 diabetes mellitus (T2DM) is a growing public health concern in Bangladesh, with an estimated 13.9 million people affected in 2024.1 Alarmingly, 43% of these individuals remain undiagnosed, especially in rural and underserved populations, where diagnostic services are limited.2–4 Many patients present with complications such as neuropathy, retinopathy and cardiovascular disease at the time of diagnosis, increasing the burden on both the health system and individual patients.5–7
Screening and early intervention have been shown to be effective strategies for reducing T2DM incidence. Major trials such as the Diabetes Prevention Program and the Finnish Diabetes Prevention Study demonstrated that lifestyle modifications and pharmacological interventions could prevent or delay the onset of T2DM in high-risk individuals.8 9 Despite the promise of these interventions, screening tools remain a challenge in low-income and middle-income countries (LMICs) like Bangladesh.
Standard diagnostic criteria for diabetes include fasting plasma glucose (FPG), the 2-hour plasma glucose (2hPG) after an oral glucose tolerance test (OGTT) and glycated haemoglobin (HbA1c), as recommended by the American Diabetes Association and the WHO.10 11 However, these tests require specialised laboratory equipment, patient compliance with fasting and trained personnel—resources that are often lacking in rural healthcare settings in Bangladesh.
HbA1c, though useful in many high-resource settings, is expensive and often not standardised in Bangladeshi laboratories. It is also influenced by several factors, including age, pregnancy, haemoglobinopathies, and ethnicity, making it unsuitable for large population screening programmes.12 13 As a result, these challenges have prompted a shift towards simpler, more accessible screening methods.
Random capillary blood glucose (RCBG) testing is widely used in outpatient clinics and community health camps across Bangladesh. It is low-cost, non-invasive and does not require fasting. Despite these advantages, RCBG has not been validated against all three standard diagnostic methods in the Bangladeshi population. Health providers often use the global threshold of ≥11.1 mmol/L, which may not be suitable for detecting asymptomatic or early-stage diabetes.
Several international studies have explored the diagnostic accuracy of RCBG. In India, a threshold of 6.1 mmol/L showed good sensitivity for diabetes detection.14 Similar observations were reported from Thailand and China, reinforcing RCBG’s diagnostic potential in different ethnic and resource settings.15 16 However, variations in cut-off points across populations highlight the need for population-specific thresholds.
To date, no large-scale study in Bangladesh has systematically evaluated the performance of RCBG in comparison with FPG, 2hPG and HbA1c using standardised diagnostic protocols in a population-based screening context. Therefore, this study aims to assess the diagnostic accuracy of RCBG and to determine an optimal cut-off value for detecting T2DM in the adult Bangladeshi population.
Methods
Study design and study site
This cross-sectional diagnostic accuracy study was conducted between May and September 2022 at 16 centres of the Diabetic Association of Bangladesh (BADAS). BADAS provides outpatient and inpatient services to approximately 12 000–15 000 individuals daily through 130 small, medium and large centres and hospitals across the country. Study centres were randomly selected from within and outside the capital, Dhaka, covering all eight administrative divisions of Bangladesh. Participants were recruited using a systematic random sampling approach, whereby every second eligible individual presenting for diabetes screening was invited to participate.
Participants and sampling procedure
The sample size was calculated based on a national prevalence of T2DM of 8.3%, as reported in the 2018 Bangladesh The WHO STEPwise approach to NCD risk factor surveillance (STEPS) survey.17 Using the standard formula for estimating proportions—n  , where n is the required sample size, Z is the Z-score (1.96 for 95% CI), P is the expected prevalence (8.3%) and d is the margin of error—a minimum sample size of 2830 was obtained. Allowing for a 10% non-response rate, the final required sample was 3113 individuals. Participants were eligible for inclusion if they were aged 18 years or older and provided written informed consent. Individuals were excluded if they had a known diagnosis of T2DM, were taking medications known to affect glucose metabolism, had any chronic illness at the time of screening, were pregnant or were unwilling or unable to provide informed consent or communicate with the study personnel.
, where n is the required sample size, Z is the Z-score (1.96 for 95% CI), P is the expected prevalence (8.3%) and d is the margin of error—a minimum sample size of 2830 was obtained. Allowing for a 10% non-response rate, the final required sample was 3113 individuals. Participants were eligible for inclusion if they were aged 18 years or older and provided written informed consent. Individuals were excluded if they had a known diagnosis of T2DM, were taking medications known to affect glucose metabolism, had any chronic illness at the time of screening, were pregnant or were unwilling or unable to provide informed consent or communicate with the study personnel.
Based on the calculated sample size, we aimed to recruit 200 participants from each of the 16 selected BADAS centres, yielding a total of 3200 participants. A systematic random sampling technique was employed, whereby every second eligible adult presenting for diabetes screening was invited to participate. Given the high patient volume at BADAS centres, the target sample size was achieved within the study period. In total, 3320 individuals were approached, of whom exactly 3200 met the eligibility criteria, provided informed consent and were included in the final analysis. A Standards for Reporting of Diagnostic Accuracy Studies (STARD)-compliant flow diagram (figure 1) illustrates the recruitment and inclusion process.
STROBE flow diagram of participant recruitment. A total of 3320 individuals were approached across 16 BADAS centres. Following the exclusion of 120 individuals, 3200 participants were enrolled using systematic random sampling (every second eligible patient) and included in the final analysis. BADAS, Diabetic Association of Bangladesh.
Data collection
Planning of the study
Prior to study initiation, an expert panel comprising an epidemiologist, diabetologist/endocrinologist, statistician and biochemist convened with the project team leader to review and refine the study design. Recommendations from this panel were incorporated into the final protocol. One physician, one laboratory technician and three volunteers were appointed at each study centre to oversee implementation. All field staff received 2 days of structured theoretical and practical training before the commencement of data collection.
Eligible participants were provided with a detailed participant information sheet and given adequate time to ask questions and clarify concerns. Informed written consent was obtained only after confirming the participant’s comprehension of the study procedures. Individuals who did not demonstrate full understanding were excluded.
Following consent, data were collected using a three-step process aligned with the modified WHO STEPS approach: face-to-face interview (Step 1), physical measurements (Step 2) and collection of biological samples (Step 3).
Fasting blood samples were collected to measure FPG and HbA1c. Participants then consumed a 75 g oral glucose solution, followed by a second blood sample collected 2 hours later for the 2hPG test. During the 2-hour interval, trained interviewers administered a structured questionnaire based on the WHO STEPwise approach to collect sociodemographic and behavioural information.
Sociodemographic variables included age (in completed years), sex (male or female), marital status (currently married, never married, divorced/separated or widowed), education level (no formal education, primary, secondary, higher secondary or graduate and above), occupation (unemployed, informal, formal or retired) and monthly household income. Residential status was defined as urban or rural using administrative classification. Family history of diabetes in first-degree relatives was recorded.
Behavioural variables included tobacco use (current, former or never), alcohol consumption (defined as any use in the past 30 days), physical activity and dietary habits (frequency of daily fruit and vegetable consumption).
Anthropometric measurements included height, weight and waist and hip circumference, recorded using standardised protocols. Blood pressure (BP) was measured using a mercury sphygmomanometer.
After the 2-hour interval, blood samples were analysed for OGTT using a calibrated glucose analyser. RCBG was measured using a portable glucometer (OneTouch Ultra II, Lifescan, Milpitas, California, USA) based on the glucose oxidase assay. RCBG testing was conducted either on the same day (between 14:30 and 19:30 pm) or the following morning (between 8:30 and 14:30 pm) using fresh capillary whole blood obtained by finger prick from the participant’s left middle finger.
Measurements of anthropometric parameters and BP
Anthropometric measurements were performed with participants wearing light clothing and no shoes. Weight was measured using electronic digital LCD scales, calibrated daily with a standard weight. Height was recorded with the participant standing erect against a flat, wall-mounted stadiometer. Waist circumference (WC) was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest and hip circumference at the widest portion of the buttocks. Both measurements were obtained using a non-stretchable measuring tape with participants in a standing position. All values were recorded to the nearest 0.1 cm, following WHO STEPS protocol. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres. Waist-to-hip ratio (WHR) was derived from waist and hip circumference measurements.
To ensure the accuracy of BP readings, participants were seated and rested for 5 min prior to measurement. BP was measured on the right arm using a mercury sphygmomanometer fitted with a standard adult cuff. Systolic BP (SBP) was recorded at the first appearance of Korotkoff sounds (phase I) and diastolic BP (DBP) at their disappearance (phase V). Readings were taken to the nearest 2 mm Hg based on the top of the mercury column.
Intraobserver variability was assessed by repeating the BP measurement on the same individual after a 5-minute interval. Interobserver variability was evaluated by having two trained observers independently measure BP within a 10-minute window. The intraobserver and interobserver coefficients of variation (CVs) were 2.6% and 3.3%, respectively.
Blood glucose estimation
On arrival, 5 mL of fasting venous blood sample was collected from each participant for measurement of FPG and HbA1c. Additionally, 2 mL of venous blood sample was drawn 2 hours after the administration of 75-gram oral glucose solution. Blood samples intended for plasma glucose analysis were collected in tubes containing sodium fluoride and potassium oxalate (1:3 ratio) and centrifuged immediately. Plasma glucose was measured using the glucose oxidase method on the Dimension RxL Max platform (Siemens AG, Erlangen, Germany).
To ensure quality control, every 10th sample was analysed again for 2hPG using the same enzymatic method. HbA1c samples were collected in ethylenediaminetetraacetic acid vials (2 mg/mL) and analysed on the same day using the Bio-Rad D-10 system (Bio-Rad Laboratories, Hercules, California, USA), which employs high-performance liquid chromatography-based ion-exchange chromatography. The analytical range was aligned with the Diabetes Control and Complications Trial and National Glycohemoglobin Standardization Program recommendations, with a reference range of 4.0–6.0%.
All glucose metres used in the study were plasma-calibrated and provided reliable readings within a haematocrit range of 30–50%, without haematocrit correction. The intra-assay and interassay CV for venous glucose ranged from 0.88% to 1.88%. The mean CV for RCBG was 4.8%. All participants were informed of their glucose results as soon as the analyses were completed.
Definition of variables
General obesity was defined as a BMI of ≥25 kg/m² for both sexes. Central obesity was defined using WC cut-offs of ≥90 cm for men and ≥80 cm for women. WHR thresholds were ≥0.90 for men and ≥0.80 for women.18 19 T2DM was defined as FPG ≥7.0 mmol/L and/or 2hPG ≥11.1 mmol/L.11 Additionally, HbA1c ≥6.5% and RCBG ≥11.1 mmol/L with symptoms were considered diagnostic for T2DM.11 Diabetes symptoms were defined as the presence of at least one classic hyperglycaemic symptom, including polyuria, polydipsia, polyphagia, unexplained weight loss or generalised weakness, consistent with WHO diagnostic criteria.¹¹ Hypertension was defined as a mean SBP of ≥140 mmHg, a DBP of ≥90 mmHg or current use of antihypertensive medication.20 Smoking status was categorised as current smoker or non/ex-smoker. Socioeconomic status was stratified into three groups based on self-reported monthly household expenditure: low (<10 000 Bangladeshi Taka (BDT); approximately USD 91), medium (10 000–20 000 BDT) and high (>20 000 BDT). Education level was categorised as no formal education (unable to read or write), undergraduate (primary to higher secondary) and graduate (college or above). Physical activity was graded on a three-level ordinal scale based on self-reported leisure-time walking duration: light (<30 minutes/day), moderate (30–60 minutes/day) and heavy (>60 minutes/day). For analysis, this was converted into a binary variable: inactive (grade 1,<30 minutes/day) and active (grades 2 and 3,≥30 minutes/day).21 22 Inadequate fruit and vegetable consumption was defined as fewer than five servings per day, in accordance with WHO STEPS guidelines. This variable was included in the composite calculation of participants with at least one non-communicable disease (NCD) risk factor.23 Residential status was classified as urban or rural based on administrative definitions.24
Statistical analysis
Continuous variables were presented as means with 95% CIs and categorical variables as percentages with 95% CIs. Differences in means between groups were assessed using the independent samples t-test, while differences in proportions were evaluated using the χ² test.
The associations between RCBG and FPG, 2hPG and HbA1c were examined using Pearson’s correlation coefficients (r) and simple linear regression analysis. Bland-Altman plots were generated to assess the mean difference (bias) and limits of agreement between RCBG and FPG, 2hPG and HbA1c measurements.
Receiver operating characteristic (ROC) curve analysis was employed to assess the diagnostic accuracy of RCBG for detecting diabetes, using the OGTT as the reference standard. ROC curves were also generated to compare the diagnostic performance of RCBG, FPG, 2hPG and HbA1c. Optimal cut-off points were determined by maximising the Youden Index.
The agreement between different diagnostic methods (RCBG, FPG, 2hPG and HbA1c) was assessed using the kappa (κ) statistic. Values of κ >0.75 were interpreted as excellent agreement beyond chance, values between 0.40 and 0.75 as fair to good agreement and values <0.40 as poor agreement.
Diagnostic test characteristics, including sensitivity, specificity, positive predictive value and negative predictive value with 95% CIs, were calculated for various RCBG, FPG, 2hPG and HbA1c cut-off points. The number needed to screen (NNS), representing the number of individuals required to be screened to detect one true case of undiagnosed diabetes, was also calculated.
All statistical analyses were conducted using three software programmes: PASW Statistics V.20 (SPSS Inc., Chicago, Illinois, USA) for data cleaning, management and descriptive analysis; Stata V.14 (StataCorp LP, College Station, Texas, USA) for regression and ROC analyses and MedCalc V.20.1 (MedCalc Software Ltd, Ostend, Belgium) for determining optimal diagnostic thresholds based on the Youden Index.
All analyses were two-sided, and statistical significance was set at p value <0.05. The findings were reported in accordance with the STARD guidelines.
Patient and public involvement
Patients and the public were not involved in the design, conduct, analysis or dissemination plans of this research.
Results
Table 1 presents the baseline characteristics of the study participants stratified by sex. The mean age of participants was 44.4 years, with females being slightly younger than males. More than half of the participants reported a family history of diabetes. Low levels of physical activity and inadequate fruit and vegetable intake were common across both sexes. Obesity, defined by BMI, was more prevalent among females, and a significantly higher proportion of females had abdominal obesity. Mean SBP and DBP were significantly higher in males. While the overall prevalence of T2DM did not differ significantly by sex, males showed higher mean FPG levels, and females reported more T2DM-related symptoms. Biochemical parameters such as 2hPG, HbA1c and RCBG were similar between sexes. A high proportion (96.0%) had at least one NCD risk factor.
Basic characteristics of the study participants by sex
Table 2 shows the correlation (p values) between RCBG, FBG, 2hAG and HbA1c. All four blood glucose tests are positively correlated. The correlation of RCBG with FPG, 2hPG and HbA1c was 0.828 (p<0.001), 0.840 (p<0.001) and 0.826 (p<0.001), respectively. The strongest linear relationship was observed between RCBG and 2hPG.
Correlation (p values) between RCBG, FBG, 2hAG and HbA1c
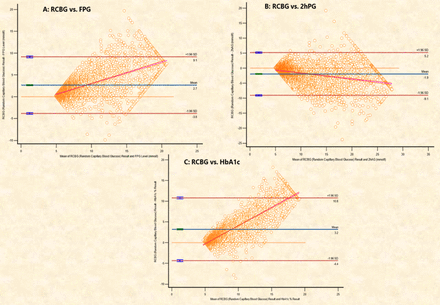
Figure 2 shows the concordance between RCBG, FBG, 2hAG and HbA1c using Bland-Altman plots. The mean differences were 2.7 mmol/L (RCBG vs FPG), 1.9 mmol/L (RCBG vs 2hPG) and 3.2 mmol/L (RCBG vs HbA1c). These results demonstrate a consistent slight positive bias in RCBG compared with the other diagnostic measures. Despite this, the narrow 95% limits of agreement indicate good concordance, suggesting RCBG as a reliable tool for diagnosing diabetes in resource-limited settings.
Bland-Altman plots showing the agreement between random capillary blood glucose (RCBG) and (A) fasting plasma glucose (FPG), (B) 2-hour plasma glucose (2hPG) and (C) glycated haemoglobin (HbA1c).
Figure 3 shows the diagnostic performance of RCBG in comparison to FPG, 2hPG and HbA1c for diagnosing diabetes. In figure 3A, the ROC curve of RCBG shows an optimal cut-off of 8.7 mmol/L with a sensitivity of 79.7%, specificity of 89.1%, AUC of 0.905 and Youden index of 0.697. Figure 3B shows ROC curves comparing the diagnostic performance of FPG, 2hPG, HbA1c and RCBG. FPG has the highest AUC (0.968), followed by 2hPG (0.964), HbA1c (0.936) and RCBG (0.905). This shows that RCBG has slightly lower diagnostic accuracy but is still a useful tool for diagnosing diabetes in resource-limited settings.
Diagnostic performance of random capillary blood glucose (RCBG) in comparison to fasting plasma glucose (FPG), 2-hour plasma glucose (2hPG) and glycated haemoglobin (HbA1c) for diagnosing diabetes.
Table 3 summarises the diagnostic performance of different tests for detecting T2DM, including FPG, 2hPG, HbA1c and RCBG using both the current (≥11.1 mmol/L) and proposed (≥8.7 mmol/L) cut-off points, with and without typical symptoms. Among all tests, 2hPG demonstrated the highest diagnostic accuracy (95.9%) and agreement (κ=0.917), followed by FPG (accuracy 92.1%) and HbA1c (accuracy 87.7%). While RCBG with the conventional cut-off had lower sensitivity (63.1%) and agreement (κ=0.611), the proposed RCBG threshold of ≥8.7 mmol/L improved sensitivity (80.4%), diagnostic accuracy (84.7%) and agreement (κ=0.695). The NNS was the lowest for HbA1c (2.36) and 2hPG (2.40), followed closely by RCBG ≥8.7 mmol/L (2.74), indicating the practical utility of the proposed threshold in population-level screening. The addition of typical hyperglycaemic symptoms marginally improved RCBG performance at both thresholds.
Comparison of diagnostic performance of FPG, 2hPG, HbA1c and RCBG (both proposed and currently used cut-off point) to diagnose T2DM
Figure 4A illustrates the mean RCBG levels among asymptomatic and symptomatic individuals, stratified by whether confirmatory testing was conducted on the same day or the next day. Among symptomatic participants, the mean RCBG level was higher when confirmatory testing occurred on the same day (11.5 mmol/L) compared with next-day testing (10.4 mmol/L). A similar trend was observed among asymptomatic individuals, though the difference was less pronounced (10.8 mmol/L vs 10.6 mmol/L).
Comparison of random capillary blood glucose (RCBG) levels in participants with and without symptoms, measured at different time points (same day vs. next day).
Figure 4B compares the diagnostic yield for T2DM across different RCBG-based criteria, also stratified by the timing of confirmatory testing. Across all cut-offs, same-day confirmatory testing resulted in a higher proportion of T2DM diagnoses compared with next-day testing. The highest detection rate (49.6%) was observed using the proposed RCBG cut-off of ≥8.7 mmol/L with symptoms, when testing was performed on the same day. This suggests that diagnostic yield may be influenced not only by glucose thresholds and symptom presence but also by the timing of diagnostic confirmation.
Discussion
This study is one of the first in Bangladesh to evaluate the diagnostic performance of RCBG against FPG, 2hPG and HbA1c in detecting undiagnosed T2DM. With a large, systematically selected sample across all eight administrative divisions, our findings not only provide a population-specific RCBG threshold but also support its practical utility in resource-constrained settings.
More than 60% of the Bangladeshi population lives in rural areas where diagnostic infrastructure for FPG, 2hPG or HbA1c is often lacking.24 In these contexts, RCBG measured by handheld glucometers is frequently the only diagnostic option. Despite this reality, limited evidence has been available to support specific RCBG thresholds tailored to local populations.
The study found a high rate of undiagnosed T2DM, ranging from 33.2% to 49.5%, as defined by different diagnostic methods including FPG, 2hPG, OGTT (both FPG or 2hPG), HbA1c and RCBG. This finding is consistent with the International Diabetes Federation (IDF)’s 45%.1 The revised RCBG threshold significantly improved the detection rate of previously undiagnosed T2DM, highlighting its potential utility for early identification and timely clinical management.
This study found strong correlations between RCBG and other diagnostic standards: 0.828 with FPG, 0.840 with 2hPG and 0.826 with HbA1c (p<0.001 for all). These findings are consistent with prior studies from India,14 Thailand15 and other LMICs, where RCBG has shown strong concordance with OGTT or laboratory-based diagnostics. In contrast to studies in high-income settings that use RCBG primarily with symptoms, our data suggest that RCBG alone—without symptom screening—can be a reliable diagnostic tool, particularly in mass screening programmes.
Previous studies conducted in various regions have reported a wide range of optimal RCBG cut-off values, typically between 5.5 and 7.9 mmol/L, depending on population demographics, clinical settings and diagnostic reference standards.14 15 25–27 Although the RCBG cut-off identified in our study (8.7 mmol/L) is higher, this variation may be attributed to the unimodal glucose distribution in our sample, the specific use of OGTT as the reference standard and differences in ethnicity and dietary patterns. Therefore, while the absolute value differs, our findings are aligned with the broader evidence supporting the utility of RCBG as a valid screening tool—particularly when population-specific validation is applied.
In addition, the RCBG cut-off value of ≥8.7 mmol/L showed a good agreement with OGTT, 2hPG and HbA1c cut-off values for diagnosing T2DM than the currently used RCBG cut-off value of ≥11.1 mmol/L. One article by Caroll et al highlighted the potential negative consequences of medical screening, mainly a false-positive result.28 This can lead to overdiagnosis and overtreatment, harming patients physically and financially. Our study showed that a value of ≥8.7 mmol/L had a 50% lower rate of false-positive cases than a value of ≥11.1 mmol/L. This indicates that the former cut-off value may be more useful in clinical practice.
Importantly, the current study demonstrates that adding the criterion of symptoms to RCBG thresholds did not improve diagnostic performance meaningfully. In fact, our data show that symptom-based diagnosis (RCBG ≥11.1 mmol/L + symptoms) had lower sensitivity and agreement (κ=0.623) than the proposed RCBG ≥8.7 mmol/L threshold alone (κ=0.695). This supports the idea that reliance on subjective symptoms may hinder early detection and should not be required for diagnosis in mass screening.
The diagnostic yield of RCBG was influenced by the timing of confirmatory testing. Same-day confirmatory testing yielded higher RCBG values and higher detection rates of T2DM, suggesting that RCBG is most effective when used during immediate screening encounters. Such operational insights are crucial for designing real-world diabetes screening programmes, particularly in community-based settings and primary care units.
In terms of predictive efficiency, RCBG performed better than expected. Our logistic regression analysis showed that RCBG ≥8.7 mmol/L had a stronger association with OGTT-defined T2DM than the conventional ≥11.1 mmol/L cut-off (OR: 8.91 vs 5.52). This reinforces the clinical relevance of the revised threshold. Furthermore, the NNS for RCBG ≥8.7 mmol/L was 2.74, closely aligning with NNS for FPG (2.86) and 2hPG (2.40), confirming its cost-effectiveness and practical relevance.
Cost analysis is an important consideration in health policy decision-making. RCBG is significantly less expensive (USD 0.18/test) than FPG (USD 2.73/test) or HbA1c (USD 5.46/test). This cost advantage is particularly compelling for LMICs like Bangladesh, where the national health budget per capita is limited. Prior economic analyses, such as those by Marley et al 29 and Meriggi et al,30 have also highlighted the economic feasibility of using RCBG for mass screening.
Furthermore, our results support the WHO and IDF’s recommendations for opportunistic screening for T2DM using affordable point-of-care tools. This study aligns with the goals of the WHO Global Action Plan for NCDs and provides actionable evidence for countries developing national diabetes screening policies. Our proposed threshold fills a critical evidence gap and presents an opportunity to guide national diabetes screening guidelines in Bangladesh and similar LMICs.
Strengths of our study include a large, nationally representative sample collected from 16 centres across all administrative divisions, ensuring geographic and demographic diversity. The use of WHO-recommended diagnostic tools (OGTT, HbA1c and FPG) as gold standards enhances the validity of the findings. Laboratory quality control was ensured through internal and external validation at BADAS laboratories. The systematic random sampling method reduces selection bias, and the standardisation of measurements further strengthens the reliability of the data. Additionally, trained clinicians and technicians from BADAS conducted the clinical and anthropometric assessments, contributing to data quality and substantial cost savings. It is worth noting that BADAS operates a comprehensive national diabetes care infrastructure, managing about 60% of diabetic patients in Bangladesh through its network of 130 diabetes centres, 350 accredited subdistrict facilities and 100 diabetes screening corners located in remote villages. This extensive, structured network contributes significantly to standardised clinical practice, quality care and reliable data collection.31
However, this study also has limitations. The data were collected at a single time point, making it a cross-sectional analysis that cannot establish causal relationships. The diagnosis of T2DM was based on a single measurement of OGTT, HbA1c and RCBG, whereas clinical practice typically requires repeat testing for confirmation. Although the study aimed to determine optimal cut-off values for diabetes diagnostic tools, it did not evaluate the ability of these methods to predict long-term diabetes-related complications. Additionally, individuals with previously diagnosed diabetes or pre-diabetes were excluded based on self-report. While self-reporting is generally reliable for identifying diagnosed diabetes, it may be less accurate for identifying pre-diabetes. The study also did not account for metabolic variability, differences in recent food intake or the inherent fluctuations in capillary blood glucose measurements, which may influence glycaemic readings. Furthermore, clinical and anthropometric assessments were conducted only once, without duplicate measurements or a second observer, increasing the potential for measurement error. Although systematic random sampling was applied across all eight administrative divisions, our recruitment exclusively from BADAS centres, which primarily serve individuals aware of their diabetes risk, might have led to overrepresentation of high-risk populations and thus potentially overestimated the diagnostic accuracy and prevalence rates. Consequently, generalising these findings to the broader Bangladeshi population or other healthcare settings should be done cautiously. Further community-based studies are recommended to confirm and extend these findings to guide policy recommendations.
In conclusion, RCBG may serve as an effective and affordable preliminary diagnostic tool for identifying T2DM, particularly in resource-limited settings. The proposed cut-off of ≥8.7 mmol/L demonstrated improved diagnostic performance compared with the currently used threshold. However, these findings should be interpreted cautiously, and further validation studies are needed to assess long-term clinical outcomes and generalisability to other populations.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request from BB, the study principal investigator.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Ethical Review Committee of BADAS (BADAS-ERC/EC 122100331). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors express their deep admiration to the BADAS authority, the Non-Communicable Disease Control (NCDC) Program of the Directorate General of Health Services (DGHS), the Ministry of Health and Family Welfare and the Japan International Cooperation Agency (JICA) for their technical support. They also express their gratitude towards all the participants and those individuals involved in this study.
References
Footnotes
Contributors BB contributed to study planning, statistical analysis plan, wrote the statistical methods section, ran the statistical analysis and wrote the manuscript. TS and SBM collected and researched the data and wrote the manuscript. TA, FA, NKQ and ASM drafted sections of the article and contributed to discussion. RI, SP, SUM, RIC, RO, DCR, SRC, SSA, SA and TA contributed to data collection and drafted sections of the article. FP, MAS, HM, MRA and AKAK reviewed/edited the manuscript. BB is the guarantor and accepts full responsibility for the work, had access to the data, controlled the decision to publish, performed grammar correction and checked for plagiarism.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.