Article Text
Abstract
Objectives Glycated albumin (GA) and body mass index (BMI) are associated with the risk of atherosclerotic cardiovascular disease (ASCVD). However, the role of BMI in the association between GA and 10-year ASCVD risk is still not fully understood.
Design A retrospective cross-sectional study.
Setting In this retrospective cross-sectional study, 4646 healthy subjects who received a full health examination at the Health Management Medical Center, Third Xiangya Hospital of Central South University, from 1 January 2022 to 30 December 2023 were initially identified. According to the exclusion criteria, 2107 participants were included in the final analysis.
Participants The inclusion criteria for this study included (a) age is ≥18 years old and (b) subjects were long-term residents of Hunan province.
Primary and secondary outcome measures The 10-year ASCVD risk was evaluated via the China-PAR equation. The link between GA and 10-year ASCVD risk was examined through a multivariable logistic regression model, and the dose–response relationship was demonstrated using the restricted cubic spline. The potential mediation effect of BMI on this association was explored, and the differences in this mediation effect across age and metabolic-associated fatty liver disease (MAFLD) subgroups were analysed.
Results Elevated GA levels were positively linked to an intensified 10-year ASCVD risk (OR=1.160, 95% CI 1.055 to 1.276). Additionally, BMI was negatively linked to GA and 10-year ASCVD risk. BMI mediated 13.9% of the connection between GA and 10-year ASCVD risk. Specifically, the mediating effect of BMI remained significant in the 40–60-year age subgroup and non-MAFLD subgroup, with mediation ratios of 43.7% and 8.5%, respectively.
Conclusions GA is a key predictor of 10-year ASCVD risk, and BMI partially mediates this relationship in healthy populations. Therefore, targeted weight management is recommended to reduce the adverse effect of GA on 10-year ASCVD risk in different populations.
- Diabetes & endocrinology
- Cardiovascular Disease
- Risk management
- Body Mass Index
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
We used a mediation model to identify body mass index (BMI) as a partial mediator in the association between glycated albumin (GA) and 10-year atherosclerotic cardiovascular disease (ASCVD) risk.
We applied multivariate logistic regression to explore the link between GA and 10-year ASCVD risk, controlling for confounding effects stepwise.
Restricted cubic spline curves were used to illustrate the dose–response association between GA and 10-year ASCVD risk.
The study’s retrospective cross-sectional design prevents establishing causality between GA and 10-year ASCVD risk and exploring the GA–BMI bidirectional relationship.
Introduction
Cardiovascular diseases (CVDs) represent the primary culprits behind deaths and disability worldwide, presenting a substantial risk to human health. This category of diseases encompasses coronary artery disease (CAD), stroke and myocardial infarction, which commonly arise due to arteriosclerosis, hypertension, hyperlipidaemia and diabetes.1–5 Currently, glycated haemoglobin A1c (HbA1c) is recognised as a leading indicator in the clinical assessment of long-term blood glucose control. The latest Kidney Disease: Improving Global Outcomes guidelines have recommended a target value of 6.5–8.0% for HbA1c in those with diabetes and chronic kidney disease (CKD) to reduce CVD risk.6 Nevertheless, HbA1c can be influenced by haemoglobin levels, red blood cell lifespan, anaemia, liver dysfunction and kidney disease.7 Concurrently, there is increasing interest in an alternative indicator, glycated albumin (GA), a novel index for glucose monitoring, reflecting average blood glucose levels over the past 2–3 weeks8 9 GA is calculated as the ratio of GA to total albumin concentration.10 Unlike HbA1c, GA has the advantage of being independent of haemoglobin and red blood cell turnover and less affected by red blood cell lifespan, thus serving as a favourable marker for assessing blood glucose levels.11
Numerous guidelines have recommended that the prevention and treatment of atherosclerotic CVD (ASCVD) be based on risk assessment. Traditional risk assessment methods, including the Framingham D'Agostino 2008, Pooled Cohort Equations (PCE) white model, Systematic COronary Risk Evaluation (SCORE) model and Quantitative Risk Index for Stroke (QRI SK) model, are recognised for their reliability in the Western populations. However, the Chinese population presents distinct disease patterns and risk factors, making these models less suitable. Particularly, the Framingham D’Agostino model overestimates the CVD risk in men and underestimates it in women, a trend similarly observed in the PCE model.12 Since 2003, several large-sample prediction models for CVD risk have been established for Chinese adults. Among them, the 10-year risk prediction models for Chinese CVD13 14 and the prediction for ASCVD risk in China (China-PAR) model for ASCVD15 are notable. The China-PAR model, a CVD risk assessment tool developed by Chinese scholars based on large-sample cohort data, is most often externally validated and is considered potentially a better option for predicting CVD risk in China.16 The China-PAR model estimates the 10-year ASCVD risk based on validated demographic, clinical and lifestyle indicators, including age, sex, family history, blood pressure (systolic blood pressure (SBP), diastolic blood pressure (DBP)), lipid profiles (total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C)), smoking, diabetes, abdominal obesity (waist circumference (WC)), urban/rural residence and regional cardiovascular epidemiology.
Obese people have a high prevalence of ASCVD, and body mass index (BMI), as an important indicator for assessing obesity in an individual, has a very close link with ASCVD risk. Similarly, the performance of GA in predicting CVD risk has attracted much attention in recent years. GA, as an early glycated protein, may be more sensitive than other markers to CVD and its complications.5 17 Several publications have found a link between GA and cardiovascular mortality in patients with diabetes mellitus (DM).9 18 19 Notably, multiple studies confirm that GA is stronger than HbA1C associated with CVD in patients with CKD requiring dialysis.18 20 21 Several papers have noted a weak negative connection between GA and BMI in DM populations but with a narrow range for BMI or GA values.11 22–27 In contrast, an investigation of Caucasian populations with a median BMI of 38.4 (large body weight) similarly unveiled a marked negative link between BMI and GA.7 Therefore, the association between GA and BMI in a wider population remains to be confirmed by further studies. There are few studies on the association among GA, BMI and 10-year ASCVD risk in normal populations. Hence, this article illustrates whether BMI mediates the link between GA and 10-year ASCVD risk in the normal population.
Methods
Participant selection
This cross-sectional study obtained data from 4646 healthy subjects during health checkups at the Health Management Medicine Center, Third Xiangya Hospital of Central South University, from 1 January 2022 to 30 June 2023. To control for potential biases, we included all health examination data during this period, rather than relying on random sampling.
The inclusion criteria for this study included (a) age is ≥18 years old and (b) subjects were long-term residents of Hunan province. The exclusion criteria of this study were (a) subjects did not have complete data required for this study; (b) subjects did not provide written informed consent; (c) subjects reported established family history and medical history of CVD and cerebrovascular diseases (including coronary heart disease, stroke, congestive heart failure, myocardial infarction and angina) in questionnaires and (d) subjects discovered severe liver or kidney dysfunction (which may affect GA results) in health checkups. According to the exclusion criteria, 84 participants under the age of 18 were excluded, 1237 participants lacking GA, BMI and China-PAR equation-related data were excluded, 276 participants with self-reported CVD and cerebrovascular diseases on the questionnaire were excluded, 28 participants with severe liver or kidney dysfunction (defined as a history of severe liver impairment, liver cirrhosis, CKD stage 3 or higher or end-stage renal disease, which may affect GA results) were excluded and 914 participants with missing information on cardiovascular risk factors were excluded. Ultimately, 2107 participants were enrolled. The specific selection process is displayed in figure 1.
Patient and Public Involvement
Patients and members of the public were engaged in the design and conduct of this study. All participants were recruited from Health Management Medicine Center of Third Xiangya Hospital of Central South University. We held discussions with 12 participant representatives to collect their insights on the research topic and desired outcomes. Their feedback was instrumental in shaping the research questions and outcome measures. During the study design phase, these representatives assisted in refining the questionnaire outline to ensure the questions were relevant to the participants' real - world situations and easy to understand. In the results interpretation phase, we gathered feedback from the participant representatives on the report format. All participants provided signed informed consent. The involvement of the participants added a valuable perspective to this study and ensured that the research was practically oriented towards the concerns of the target group.
Flowchart for study population selection. BMI, body mass index; GA, glycated albumin.
Measurements
GA measurement
Compared with HbA1c testing, the detection methods for GA are not yet fully standardised. In this study, ELISA, a widely adopted and reliable technique,28 was employed to detect GA.
Cardiovascular risk estimation based on the China-PAR project
The China-PAR score was employed to appraise ASCVD risk. The China-PAR model encompassed sex, age, SBP, TC, HDL-C, WC, smoking status, diabetes, region, urbanisation and family history of ASCVD. The participants were allocated into three risk subgroups based on the scores: <5% as low risk, 5–10% as medium risk and ≥10% as high risk.15
Health status and lifestyle information of the participants were collected through questionnaires during their health checkups. This questionnaire, designed based on the National Physical Examination Questionnaire,29 included details on the participants’ and family history of CVDs and cerebrovascular diseases, and lifestyle information such as history of hypertension, CVDs, DM, smoking, alcohol consumption and physical activity. Hypertension was diagnosed as SBP≥140 mm Hg and/or DBP≥90 mm Hg or receiving antihypertensive treatment. Diabetes was diagnosed as fasting plasma glucose≥7.0 mmol/L, HbA1c≥6.5% or receiving hypoglycaemic treatment. A family history of ASCVD was described as congestive heart failure, stroke, coronary heart disease, myocardial infarction or angina in first-degree relatives. Based on the Chinese guideline on the primary prevention of CVDs,30 which prioritises actionable thresholds for public health interventions, participants were allocated into a medium-high risk group and low-risk groups in this study.
Covariate assessment
Demographic data were obtained from physical examination reports. Age as a continuous variable was categorised into subgroups: 18–39 years, 40–60 years or >60 years. During the physical examination, weight, height, WC, SBP and DBP were gauged. BMI was presented as weight (kg) divided by height square (m²). Laboratory tests covered routine blood tests, liver function tests, GA, fasting blood glucose (FBG), HbA1c, fasting insulin and blood lipids. It should be noted that GA rather than HbA1C was the primary focus of this study, and most research subjects did not have both measurements above. HbA1C was not set as a mandatory enrolment criterion. All participants underwent liver and cervical vascular ultrasonography performed by an ultrasonologist after an overnight fast. These reports were reviewed and confirmed by another senior ultrasonologist. The diagnoses of fatty liver (hepatic steatosis) and cervical vascular plaques were based on liver and cervical vascular ultrasound scans using linear array or convex high-frequency probes with frequencies of 5–10 MHz. These scans evaluated the echo, size and morphology of the liver, as well as intimamedia thickness, plaque size, morphology and location of cervical vessels. Furthermore, medication history, smoking history, alcohol consumption and exercise frequency were recorded directly from the physical examination questionnaire. Medication history in this study was defined as medication used for DM, hypertension and dyslipidaemia. Smoking history was defined as smoking continuously or cumulatively for more than 6 months.29 Alcohol consumption manifested as consuming over 10 g of alcohol per day.31 Physical activity represented moderate-intensity or high-intensity physical activity at least three times per week, with each session lasting at least 30 min.32
Diagnosis of metabolic-associated fatty liver disease
According to Eslam et al,33 metabolic-associated fatty liver disease (MAFLD) means steatosis in combination with metabolic dysfunction in the International Expert Consensus Statement on MAFLD. The diagnostic criteria included hepatic steatosis through histology (liver biopsy), imaging techniques, or blood-based biomarkers and at least one of the following conditions: overweight/obesity, type 2 diabetes mellitus (T2DM), and metabolic dysfunction.T2DM is the chronic metabolic disorder characterized by insulin resistance and impaired insulin secretion, diagnosed by: (1) fasting blood glucose ≥7.0 mmol/L, or 2 hours blood glucose ≥11.1 mmol/L during 75g oral glucose tolerance test (OGTT), or HbA1c ≥6.5%, or current use of antidiabetic medication. Metabolic dysfunction refers to at least two of the seven metabolic risk abnormalities (figure 2). In this study, all subjects were from a health screening cohort. Thus, balancing feasibility, cost-effectiveness and diagnostic accuracy in the screening population, we uniformly used liver ultrasonography after an overnight fast to assess hepatic steatosis in all subjects, instead of other imaging techniques (eg, vibration-controlled transient elastography or MRI elastography), blood-based biomarkers, such as Fibrosis-4 score (FIB-4) and Aspartate Aminotransferase-to-Platelet Ratio Index (APRI), or liver biopsy.
Diagnostic standard flowchart of metabolic-associated fatty liver disease. BMI, body mass index; HDL, high-density lipoprotein cholesterol.
Data analysis
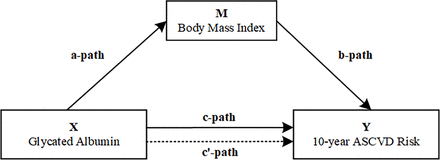
Continuous variables were denoted as median and interquartile spacing and compared using the independent-samples t-test. Categorical variables were depicted as unweighted frequencies, weighted percentages and SE, and compared using the χ2 test. Multivariate logistic regression analysis was performed to assess the association between GA (a continuous exposure variable) and 10-year ASCVD risk (a binary outcome variable). Variables showing between-group differences in the baseline information table (p<0.05) were identified and included as potential confounders, serving as covariates in subsequent analyses. Given the strong association of fasting glucose and HbA1c with the 10-year ASCVD risk, they were excluded from the logistic regression model. Three models were constructed to progressively control for the effects of confounders: unadjusted crude model 1; model 2 adjusted for age, sex, smoking status, sport status, BMI, WC, SBP and DBP; and model 3 adjusted for all covariates, such as albumin, platelet, TC, triglyceride, HDL-C, cervical vascular plaques and MAFLD. Subsequently, restricted cubic spline (RCS) analysis was adopted to demonstrate the dose–response relationship between GA and 10-year ASCVD risk. Finally, a logistic regression model was employed to illustrate the relationships among GA, BMI and 10-year ASCVD risk. To analyse the mediation effect of BMI [(mediation effect/total effect) × 100%], a simple mediation model was used, with three paths (figure 3). The total effect represented the impact of GA (exposure) on 10-year ASCVD risk (outcome). Path A assessed the effect of GA on BMI (mediator). Path B evaluated the link between BMI and 10-year ASCVD risk. Path C estimated the direct impact of GA on 10-year ASCVD risk. The mediated effect was calculated as (mediated effect/total effect) × 100%.
Path diagram of the mediation analysis models. ASCVD, atherosclerotic cardiovascular disease.
All statistical analyses, including mediation analysis, were performed in the R statistical package. Statistical significance was delineated at a two-sided p-value of <0.05.
Results
Characteristics of the participants
Online supplemental table S1 displays the baseline characteristics of the study participants. Based on the three risk categories mentioned above, participants were assigned to a medium-high-risk group (n=230) and a low-risk group (n=1877). The differences were statistically notable (p<0.001) in age, BMI, WC, SBP, DBP, albumin, GA, platelet, FBG, TC, cervical vascular plaques, smoking status and MAFLD.
Supplemental material
Online supplemental table S2 exhibits the multivariate logistic analysis results. In model 1, a higher GA was greatly associated with higher odds of 10-year ASCVD risk (OR 1.138, 95% CI 1.094 to 1.185). After adjusting for covariates (age, sex, smoking status, sport status, BMI, WC, SBP, DBP, albumin, platelet, TC, triglyceride, HDL-C, cervical vascular plaques and MAFLD), GA was positively associated with 10-year ASCVD risk (OR 1.160, 95% CI 1.055 to 1.276) in model 3.
Supplemental material
The RCS curve showed that increased GA levels were significantly linked to an increased 10-year ASCVD risk (p for overall<0.001), with a linear relationship (p for non-linear=0.2616) (figure 4). The red solid line indicates the point estimate of OR, and the pink shaded area represents the 95% CI. The curve indicates that when GA levels are below 12.95%, the OR remains around 1, suggesting that GA does not significantly affect the risk within this range. However, when GA levels>12.95%, the OR starts to increase significantly, indicating a marked association between higher GA levels and greater 10-year ASCVD risk.
Restricted cubic spline curve for association between OR of glycated albumin and 10-year atherosclerotic cardiovascular disease risk.
Mediation analysis of BMI
We then delved into the mediation effect of BMI on the link between GA and 10-year ASCVD risk. All mediation analyses were carried out after adjustment for age, sex, smoking status, sport status, BMI, WC, SBP, DBP, albumin, platelet, TC, triglyceride, HDL-C, cervical vascular plaques and MAFLD.
GA was negatively associated with BMI (p<0.001) and positively associated with 10-year ASCVD risk (p<0.001). BMI was positively related to 10-year ASCVD risk (p<0.001) (online supplemental table S3). It is estimated that BMI mediated 13.9% of the total link between GA and 10-year ASCVD risk.
Supplemental material
Given the effect of age and MAFLD, subgroup analyses were implemented (online supplemental table S4). In the age group, BMI partially mediated the link between GA and 10-year ASCVD risk. In the age subgroup 40–60 years, there was a 43.7% mediating effect of BMI on the link between GA and 10-year ASCVD risk. However, in the age subgroup<40 years and >60 years, no mediating effect of BMI was observed on the association between GA and 10-year ASCVD risk. Similarly, BMI mediated 8.5% of the total link between GA and 10-year ASCVD risk in the non-MAFLD group. However, the mediating effect was not observed in the MAFLD group.
Supplemental material
Sensitivity analysis
In sensitivity analyses, we adjusted for three medication status variables (antidiabetic, antihypertensive and lipid-lowering drugs) as potential confounders. After excluding 23 cases with missing data, the analysis of 2084 eligible participants demonstrated that the positive association between GA and 10-year cardiovascular risk remained highly consistent (OR 1.153, 95% CI 1.033 to 1.277; online supplemental table S5). Detailed results of the adjusted model are provided in online supplemental table S5. This finding further supported the robustness of our primary analysis, indicating that GA maintained its independent predictive value for 10-year cardiovascular risk even after controlling for the influence of pharmacological interventions.
Supplemental material
Discussion
This study elucidated the association among GA, BMI and 10-year ASCVD risk in the normal population undergoing physical examination and estimated the mediating role of BMI. This is the first study on their associations, and our results confirm that (a) BMI mediates 13.9% of the association between GA and 10-year ASCVD risk; and (b) the mediating role of BMI varies by age and MAFLD subgroups. BMI plays a partially mediating role of 8.5% in the 40–60-year age group and no mediating role in the 30–44-year and over 60-year age groups. Similarly, BMI has an 8.5% partial mediating effect on the non-MAFLD group, but no mediating effect was revealed in the MAFLD group.
The present study examined a real-world normal population undergoing medical examinations and showed a positive association between GA and 10-year CVD risk. The association remained after adjustment for established cardiovascular risk factors and socio-behavioural factors. Although GA is widely recognised to reflect blood glucose levels, relevant studies have focused on its application in diverse situations (eg, anaemia, abnormal liver function and renal disease) where HbA1c accuracy may be impaired. However, there is controversy about the ability of GA to predict long-term ASCVD outcomes. Previous studies have manifested that GA upregulation is positively linked to CAD and its severity in DM patients, whereas HbA1c levels are not as strongly correlated with CAD.34 35 Similarly, several publications have highlighted that after adjustment for HbA1c levels, the higher GA in dialysis-requiring patients with CKD and DM, the higher risk of cardiovascular mortality, all-cause mortality, CAD, major adverse cardiovascular events, and stroke, no association was found in non-DM individuals.9 18 19 Zhao et al found in the meta-analysis that GA had a stronger association with CVD outcomes (including cardiovascular mortality in non-dialysis patients and all-cause mortality in patients undergoing dialysis) than HbA1c, and its link with major adverse cardiovascular and cerebrovascular events (MACCE), a composite endpoint of cardiovascular - related events including cardiovascular death, myocardial infarction, and stroke, was independent of traditional risk factors and HbA1c levels.36 However, Copur et al revealed a prominent association of GA levels with all-cause mortality in patients with DM undergoing dialysis, but not with CVD mortality.37 Additionally, high GA levels were independently linked to unfavourable intermediate-term efficacy in low-risk populations undergoing percutaneous coronary intervention, but the prognostic role was only present in the DM subgroup, and in the non-DM individuals, this association was not supported by clear evidence.38 It is well known that atherosclerosis is the most important cause of CVDs.39 Significant glycaemic excursions or fluctuations potentiate oxidative stress and drive atherosclerosis, which can lead to ASCVD.40 41 We hypothesised that relative to HbA1c GA may better indicate glycaemic fluctuations (eg, acute hyperglycaemia or significant postprandial glycaemic excursion) before the onset of acute coronary syndrome (within a short period),40 42 thereby promoting oxidative stress and accelerating atherosclerosis in both DM and non-DM subjects.43 44 In non-DM subjects, short-term glycaemic fluctuations, especially significant postprandial glycaemic excursion, may be manifested as small but notable differences in GA. The effect of HbA1c on non-DM participants may be confounded by non-glycaemic factors, like abnormalities in liver and kidney function and differences in red blood cell lifespan. In summary, studies comparing GA and HbA1c for long-term ASCVD prediction had mainly focused on diabetic patients and those with renal dysfunction, and there had been a lack of research on the longitudinal comparison of GA and HbA1c changes in the normal population. Therefore, further investigations are warranted to elucidate the complex pathophysiological mechanisms that link GA to ASCVD risk.
Our experimental results showed that GA was negatively associated with BMI. Research in normoglycaemic populations, patients with pre-DM and patients with T2DM has reported a link between BMI and HbA1c or GA/HbA1c.27 45 However, studies on the link between GA and BMI are very limited in normal populations in China. As mentioned earlier, several papers have demonstrated a weak negative connection between GA and BMI among DM populations, but mainly in normal-weight subjects with little fluctuation in BMI,11 22–27 or Caucasian populations with large body weights (mean BMI of 38.4). In contrast, the two papers that did not discover a negative association between GA and BMI were conducted in the Japanese population with type 1 DM with a mean BMI of 20.146 or without DM with a nearly constant GA.47 Thereby, the negative link between BMI and GA may not be exclusively attributable to glycaemic exposure, but there are other factors. An increase in BMI increases renal blood flow, glomerular filtration rate and tubular reabsorption, resulting in glomerular enlargement and obesity-associated glomerular disease.48 49 There is a difference in the rate of GA synthesis in participants with higher BMI and normal BMI.22 BMI may affect GA clearance. A mild increment in BMI is related to an increased prevalence of microalbuminuria, which is a marker of early renal damage and is also closely related to cardiovascular prognosis.50 Elevated GA levels may reflect the presence of microalbuminuria and cardiovascular events, but the exact mechanism is currently unknown. Therefore, future studies should refine the proteinuria and renal clearance of GA, which may influence the relationship between GA and BMI. Additional research using labelled albumin in animal models of obesity and DM may offer valuable insight into this process.
Interestingly, the mediation analysis unravelled that BMI mediated 13.9% of the link between GA and 10-year ASCVD risk. This result emphasises the importance of reducing BMI in lowering 10-year ASCVD risk, especially in high-GA populations, regardless of DM. Overweight and obesity are drivers of insulin resistance.51 52 Insulin resistance is the core connecting several events of cardiometabolic disorders,51–56 molecularly due to impaired insulin signalling transduction via the PI3K pathway and intact signalling transduction via the MAPK pathway, leading to altered glucose-insulin homeostasis and increased glucose fluctuations. Significant shifts in blood glucose values facilitate oxidative stress and atherosclerosis.40 41 Our study results supported this hypothesis. Although antihypertensive, lipid-lowering and antidiabetic drugs might influence ASCVD risk through different pathways, sensitivity analysis showed that GA’s independent predictive value remained unweakened after adjusting for these clinical interventions. Similarly, elevated serum GA levels are notably related to augmented carotid intimamedia thickness and vascular endothelial dysfunction, a sign of early atherosclerosis.57 Vascular smooth muscle cell proliferation, vascular endothelial dysfunction and overproduction of collagen and inflammatory cytokines contribute to atherosclerosis and adverse cardiovascular events. Patients in the high GA group were more susceptible to metabolic disorders and severe CAD than those in the low and medium GA groups and therefore had an intensified risk of CAD. When GA levels are substantially elevated (in the higher range, eg, more than 17.1%), the CAD risk augments by approximately twofold.38 Akane Mihara et al disclosed findings from a 10-year follow-up of the Hisayama study, suggesting that the highest quartile of GA levels (≥15.7%) was connected with a 2.2-fold increase in CAD incidence and a 2.5-fold increase in stroke risk.58 The exact mechanism of the mediating effect of BMI is currently unclear and may be different in different populations. Additional research is warranted to illustrate the mechanisms.
Interestingly, we found age and MAFLD were involved in the mediating role of BMI in the association between GA and 10-year ASCVD risk. Age-stratified subgroup analyses found that BMI did not mediate the association in the <40-year and >60-year groups. This finding suggests that BMI reduction in the <40-year and >60-year groups with increased GA may not be the cause for reducing 10-year CVD risk compared with the partial mediation effect in the 40–60-year group. We suggest that in the 40–60-year group, BMI reduction will be the focus of reducing the 10-year CVD risk induced by increased GA. In addition, subgroup analyses by MAFLD discovered that the link between GA and 10-year CVD risk was partially mediated by BMI in the non-MAFLD group, whereas BMI had no mediating effect in the MAFLD group. This finding suggests that BMI reduction in the MAFLD group with increased GA may not be the cause for reducing 10-year CVD risk. In the non-MAFLD group, BMI reduction will be the focus of preventing 10-year CVD risk. Since GA is an indicator of short-term glucose fluctuations and insulin resistance is the main mechanism of MAFLD, it is primarily a long-term effect. Therefore, long-term monitoring of GA is of limited benefit in assessing 10-year CVD risk in MAFLD populations. This may provide new insights into assessing and reducing 10-year CVD risk in these two groups.
Our study first investigates the mediating role of BMI in the link between GA and 10-year ASCVD risk. Nevertheless, we must acknowledge certain inherent limitations of our study. First, multiple multivariate models were adopted to unveil the independent link between GA and 10-year ASCVD risk. Essential confounding factors like alcohol consumption and smoking were obtained through self-reported data, which are potentially influenced by recall bias and misinterpretations and potentially affect the accuracy of our findings. Second, it is crucial to analyse the association between GA and proteinuria. However, since this study involved non-hospitalised populations and the incidence of proteinuria among participants was exceedingly low, statistical analysis was not possible. This limitation underscores further investigations into the renal clearance of proteinuria and GA, which is pivotal in elucidating the link between BMI and GA. Lastly, given the cross-sectional nature, it is not feasible to establish the exact causality as well as the bidirectional association between GA and 10-year ASCVD risk. Therefore, prospective cohort studies are imperative to further unveil this relationship. Despite these limitations, our study, based on a real-world context and substantial sample size, has fully adjusted for confounders, so the conclusions drawn are relatively reliable.
In conclusion, our cross-sectional study of 2107 participants demonstrates for the first time that BMI effectively mediates the impact of GA on the 10-year ASCVD risk. Consequently, healthy populations, especially those aged 40–60 without MAFLD, should realise the beneficial effects of GA in reducing their 10-year ASCVD risk through lowering BMI, rather than just preventing diabetes. Prospective studies are crucial to validate these findings and elucidate intrinsic mechanisms, thus paving the way for targeted therapeutic strategies.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants, and this retrospective study adhered to the institution and national research committee’s ethical standards. Approval was obtained from the institutional ethics committee of the Third Xiangya Hospital of Central South University and review board (ethics approval number: Quick24559). Participants gave informed consent to participate in the study before taking part.
References
Footnotes
Contributors All authors contributed to the study conception and design, commented on the previous versions of the manuscript and read and approved the final manuscript. Writing—original draft preparation: all authors. Writing—review and editing: XZ. Conceptualisation: YH and N-NC. Methodology: SX and YZ. Formal analysis and investigation: XZ and YH. Funding acquisition: ML and XZ. Resources: XZ. Guarantor and supervision: ML.
Funding This work was supported by grants from the National Natural Science Foundation of China (82304171) and the Hunan Province Natural Science Foundation (2022JJ40668); National Key Clinical Specialty Scientific Research Project (Z2023058) and Chinese Cardiovascular Association-ASCVD Fund (2023-CCA-ASCVD-018).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.