Article Text
Abstract
Purpose The Dutch Head and Neck Audit–Oral Cavity (DHNA-OC) cohort was collected to study the quality of care, current treatment and survival for oral cavity cancer (OCC) across all hospitals treating head and neck cancer (HNC) in the Netherlands.
Patients The DHNA-OC is a registry-based national cohort of 2545 first primary OCC patients treated with curative intent between 2018 and 2021. All 14 HNC hospitals in the Netherlands contributed, guaranteeing national coverage. The DHNA-OC cohort is an elaborate dataset including variables on patient and tumour characteristics, treatment, complications, recurrence rates and survival.
Findings to date The median age at diagnosis was 67 years and most tumours were early stage (cT1 in 32% and cT2 in 31%). Tongue tumours were most common, and surgery was performed in 91.3% of the patients. The number of included patients per hospital varied from 82 to 367. The proportion of advanced tumour stage varied significantly between hospitals. Substantial data completeness was acquired with only two variables exceeding 10% missing (comorbidities and performance score).
Future plans The DHNA-OC cohort will be used to study benchmarking of and current knowledge gaps in OCC care. Collaboration with other institutions or national/regional databases is highly encouraged. Some examples of planned studies are the assessment of hospital variation in outcome indicators for surgery and population-based treatment effects. The results of these studies will be used to identify best practices and continue improving the quality of care. Longitudinal cohort follow-up and enrolment will continue prospectively.
- ORAL & MAXILLOFACIAL SURGERY
- Head & neck tumours
- Head & neck surgery
- ONCOLOGY
Data availability statement
Data are available upon reasonable request. The authors welcome and encourage research collaborations using the DHNA-OC cohort, and researchers interested in collaborating on the cohort are welcome to contact the research group. Data requests will be handled by PRISMA, the scientific advisory committee for research in head and neck cancer in the Netherlands (https://iknl.nl/kankersoorten/hoofd-halskanker/onderzoek/prisma )
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The main strength of the Dutch Head and Neck Audit–Oral Cavity (DHNA-OC) cohort is its nationwide inclusion, facilitated by population-based registries that are centrally managed.
The DHNA-OC cohort is an elaborate dataset including variables on patient and tumour characteristics, given treatment, treatment complications, recurrence rates and survival.
The main limitations are the lack of data on socioeconomic status, education level and medication use.
Though all registrars adhere to the same manual and openly discuss questions when registering, variation in the interpretation of variables could exist.
Introduction
The Dutch Head and Neck Audit–Oral Cavity (DHNA-OC) cohort was designed to study current treatment, survival and quality of care for oral cavity cancer (OCC). With an incidence of ~1000 in 2023, OCC is a relatively rare cancer in the Netherlands.1 Despite the low incidence, OCC patients often require highly complex multidisciplinary integrated care.2 As in other cancers with low incidence rates, clinical trials in head and neck cancer (HNC) struggle to enrol enough participants. Therefore, real-world data are increasingly used to answer current knowledge gaps in clinical practice guidelines.
The DHNA was established in 2014 to monitor and benchmark the quality of HNC nationally.2 Auditing has been identified as an effective tool in improving the quality of care for surgical oncological fields, such as in the Dutch Surgical Colorectal Audit.3 4 By effective auditing and collaboration, the patient pathways were standardised, complication rates declined and even mortality rates decreased.4 Over the past years, DHNA data availability has improved, yet data are missing on crucial variables. To follow the lead of the colorectal audit, the DHNA-OC cohort was instigated.
Research questions that motivated the DHNA-OC cohort revolve around enhancing the quality of care and addressing current knowledge gaps. To study hospital variation, we first aim to develop a case-mix model for OCC.5 This will enable us to investigate variation in surgical complications, resection margins and textbook outcome.6 Furthermore, the indication and value of adjuvant therapy in case of a resection margin of 1–5 mm remain unclear.7 Also, debate is ongoing regarding the use of elective neck dissection versus sentinel lymph node biopsy in early-stage OCC.8 Through the DHNA-OC, we aim to offer insights derived from real-world data, contributing to enhancing new clinical practice guidelines, which currently may lack a scientific foundation.
Cohort description
This research proposal was reviewed by the Institutional Research Review Board, Erasmus Medical Center (Rotterdam, the Netherlands), and the board confirmed that the rules laid down in the Medical Research Involving Human Subjects Act do not apply to this research proposal (MEC-2022–0816).
Cohort design
The DHNA-OC is based on data from the DHNA. HNC care in the Netherlands is centralised in 14 devoted hospitals: eight head and neck oncological centres (HNOCs) and seven preferred partner hospitals (PPs).9 HNC care is covered by the Dutch health insurance system, which is obligatory and socialised. The DHNA gained national coverage in 2019 and participation is mandatory. All patients with a first primary head and neck tumour are prospectively included. Patients with in situ carcinoma, a second primary tumour, recurrent HNC, melanomas, cutaneous malignancies, thyroid carcinomas, sarcomas, neuroendocrine cancers and haematological malignancies are currently not included in the DHNA. Data are collected by trained registrars, physician assistants and administrative nurses employed by the HNC hospital or the Netherlands Comprehensive Cancer Organisation (IKNL). The complete DHNA data dictionary can be accessed online.10 The DHNA is one of 26 quality registries maintained at the Dutch Institute for Clinical Auditing (DICA).11 This institution guarantees data quality through annual data verification processes.12
Patient and public involvement
Patients were involved in the design of the DHNA.2 Patients or the public were not involved in the planning or design, recruitment or conduction of this cohort.
Participants
Data completeness is essential for reliable population-based research and evaluation of quality of care. Patients were selected from the DHNA based on the pathological conformation (biopsy) date between 1 January 2018 and 31 December 2021. Included ICD-O-3 codes for OCC were C00, C02-C04, C05.0, C5.8–9 and C06.0–8.13 Patients of ≥18 years were selected if treated with curative intent in one of the 14 HNC hospitals during the study period. Missing variables in the DHNA cohort were complemented with data from the Netherlands Cancer Registry (NCR). This is the national registry on malignancies in the Netherlands.14 Since 1989, IKNL has objectively registered all newly diagnosed patients in the NCR. Patients are assigned a unique uniform resource identifier (URI) in the treating hospital. DHNA and NCR data were matched on date of birth, hospital-URI and treating hospital. The complemented dataset was returned to the individual hospitals to retrieve the remaining missing values from electronic patient files. A head and neck surgeon or clinical HNC researcher then executed data curation. The final dataset was delivered to update the DHNA dataset with missing values.
Variables and data management
Online supplemental file 1 gives a complete overview of the DHNA-OC dataset. Comorbidity was scored using the ACE-27, and the TNM classification followed the 8th edition of the Union for International Cancer Control TNM Classification.15 16 Clinical TNM stage 0 was included in OCC cases with cTx/T0/TisN0M0 classification that were upstaged on pathological examination to pT1/T2/T3/T4. Surgical 30-day complications were classified utilising the Clavien-Dindo classification.17 Follow-up started on the date of last treatment (surgery, systemic therapy or radiotherapy). Follow-up was censored 2 years after the date of the last treatment. As the DHNA is a prospective database, a 5-year follow-up will be registered yearly (data for 2018 in 2025, 2019 in 2026 and so on). As this study included national data, a sample size calculation was deemed unnecessary.
Supplemental material
To guarantee patient privacy and Dutch privacy regulations, DICA works with a third-trusted party: Medical Research Data Management (MRDM), Deventer, the Netherlands (NEN 7510:2011 and ISO 27001:2013 certified).18 MRDM designs, develops and manages registration systems for DICA’s quality registrations, among others. MRDM processes the data from the hospital so that DICA receives only coded (pseudonymous) data. Hospitals sign an agreement with DICA and MRDM to process their data and deliver data manually (survey) or via batch. DICA’s privacy committee guarantees that data handling complies with the Dutch Personal Data Protection Act. Statistical analyses are performed in protected digital areas and cannot be traced back to specific subjects.
Findings to date
A total of 2545 patients were included (figure 1). The final DHNA-OC cohort baseline characteristics are presented in table 1.
Flow chart for inclusion in the Dutch Head and Neck Audit–Oral Cavity (DHNA-OC) cohort.
Demographic characteristics of patients included in the DHNA-OC cohort
The median age was 67 years (IQR 59–75) and 46% were females. Body mass index (BMI in kg/m2) was unknown in 2.8%, with 77% of the patients at a BMI between 18.5 and 30 kg/m2. Most patients were current smokers or drinkers (37% and 67%, respectively) with missing data on smoking and drinking history in 4.3% and 8.6%, respectively. Data on comorbidities were missing in 44%, leaving grade 0 as the most observed ACE27 score (28%). A WHO-performance score of 0 was most seen in the cohort (50%), though data were missing in 22%.
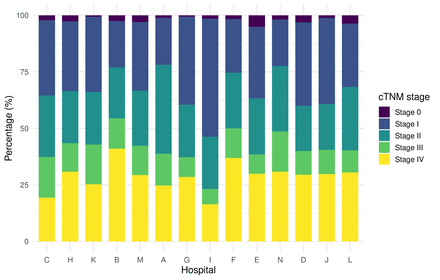
Ninety-one per cent of the tumours were squamous cell carcinoma and most were located in the tongue (43%). Clinical TNM-stage 0 tumours were present in 2.1%, stage I in 31%, stage II in 25%, stage III in 13% and stage IV in 28%. Surgery alone was performed in 57% of the patients. Surgery was complemented by radiotherapy in 28% and by chemoradiation in 7.3%. Only 4.4% received radiotherapy as definitive treatment. Seventy-six percent was treated in one of the HNOCs, and the annual inclusion rate was constant over the years. The number of patients that were included per hospital varied from 367 to 82 (figure 2). The proportion of stage III-IV tumours varied significantly between treating hospitals (p value<0.001) but was not directly proportional to hospital volume (figure 3). Overall, high data completeness was achieved, especially regarding treatment and outcome variables.
Number of patients curatively treated for first primary oral cavity cancer in the 14 head and neck oncology hospitals in the Netherlands between 2018 and 2021 (n=2545).
Tumour stage for first primary oral cavity cancer patients curatively treated in the 14 head and neck oncology hospitals in the Netherlands between 2018 and 2021 (n=2545).
2-year follow-up indicated 76% of the patients alive without and 2.5% of the patients alive with disease (figure 4). Follow-up data was missing in 78 patients (3.1%). Of the deceased patients (n=462), the cause of death was unknown in 36% (n=167). The remaining patients died of disease (7.4%), other causes (3.8%) or treatment complications (0.4%). The capture rate of the DHNA-OC cohort is compared with the annual incidence rate for OCC registered in the NCR in figure 5. The difference in annual inclusion between the NCR and DHNA-OC cohort can be attributed to DHNA exclusion criteria. The DHNA excludes patients receiving no treatment, primary palliative treatment and patients diagnosed with second primary OCC, melanoma and lip tumours.
Kaplan-Meier curve for 2 year overall survival.
Inclusion of Dutch Head and Neck Audit–Oral Cavity cohort (blue) compared with the oral cavity cancer incidence (purple line) in the Netherlands between 2018 and 2021.
As the DHNA is a prospective database, future OCC patients will be added to the DHNA-OC cohort. The authors welcome and encourage research collaborations using the DHNA-OC, and researchers interested in collaborating on the cohort are welcome to contact the research group. Data requests will be handled by PRISMA, the scientific advisory committee for research in head and neck cancer in the Netherlands (https://iknl.nl/kankersoorten/hoofd-halskanker/onderzoek/prisma).
Strengths and limitations
The DHNA-OC cohort is an elaborate dataset including variables on patient and tumour characteristics, given treatment, treatment complications, recurrence rates and survival. As DHNA-OC data are population-based, the generalisability of future study results is facilitated. Considerable data completeness has been acquired compared with previous research. The only variables with >10% missing or unknown values were the ACE27 score (44%) and the WHO performance score (22%). Described OCC cohorts in the literature are mostly based on declaration data, lack national coverage, are completely retrospectively collected, or pool data for different HNC subsites.19–24
The main limitations are the lack of data on socioeconomic status, education level and medication use. These variables are currently not included in the DHNA, but DICA is working on implementing links with other databases to expand the DHNA. However, strict Dutch privacy laws complicate linking processes. Though all registrars adhere to the same manual and openly discuss questions when registering, local variation in the interpretation of variables could exist. Annual numbers for the DHNA-OC cohort are lower compared with the OCC incidence rate in the Netherlands during the study period in the NCR (figure 5). This can mostly be explained by the exclusion of second primary tumours, cutaneous malignancies and palliative patients in the DHNA-OC. Taking these exclusions into account, we believe a reliable sample size has been acquired.
Data availability statement
Data are available upon reasonable request. The authors welcome and encourage research collaborations using the DHNA-OC cohort, and researchers interested in collaborating on the cohort are welcome to contact the research group. Data requests will be handled by PRISMA, the scientific advisory committee for research in head and neck cancer in the Netherlands (https://iknl.nl/kankersoorten/hoofd-halskanker/onderzoek/prisma )
Ethics statements
Patient consent for publication
Ethics approval
This research proposal was reviewed by the Institutional Research Review Board, Erasmus Medical Center (Rotterdam, the Netherlands) and the board conforms that the rules laid down in the Medical Research Involving Human Subjects Act do not apply to this research proposal (MEC-2022-0816).
Acknowledgments
The authors thank all Dutch Head and Neck Audit Oral Cavity Collaborator Group members for contributing. The authors thank all registrars, physician assistants and administrative nurses who registered patients in the Dutch Head and Neck Audit. The authors thank the Netherlands Comprehensive Cancer Organisation (IKNL) registration team for collecting data for the Netherlands Cancer Registry. This work was supported by the Department of Otorhinolaryngology and Head and Neck Surgery of the Erasmus Medical Centre Cancer Institute (Rotterdam, the Netherlands).
Footnotes
Collaborators Dutch head and neck audit - oral cavity collaborator group: Robert JJ van Es, Guido B van den Broek, Robert P Takes, Gyorgy B Halmos, Dominique VC de Jel, Richard Dirven, Martin Lacko, Lauretta AA Vaassen, Jan-Jaap Hendrickx, Marjolijn AE Oomens, Hossein Ghaeminia, Jeroen C Jansen, Annemarie Vesseur, Rolf Bun, Leonora Q Schwandt, Christiaan A Krabbe, Thomas JW Klein Nulent, Alexander JM van Bemmel, Reinoud J Klijn.
Contributors HDvO: ethical permission, statistical analysis. HDvO, JAH and RJBdJ: drafting of the manuscript. RJBdJ: initiator and guarantor. All authors: conceptualisation, data extraction, data review, interpretation of the results, manuscript review, approval and reading of the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.