Article Text
Abstract
Introduction Elderly patients are known to be vulnerable to postoperative pulmonary complications (PPCs), especially pneumonia. Apart from elder age, preoperative pulmonary diseases, anaemia, malnutrition, dysphagia and frailty may all be contributing factors to PPCs. Poor oral hygiene is a risk factor for PPC as well, as oropharyngeal microflora might be introduced to the lower respiratory tract following endotracheal intubation for general anaesthesia during surgery. Immune regulation, nutrition supplementation and improvement of oropharyngeal microflora might regulate immune and stress response and can be beneficial to elderly patients exposed to surgical stress. In this study, we will explore the effects of perioperative oral decontamination and immunonutrition supplementation on the incidence of postoperative pneumonia in high-risk elderly surgical patients.
Methods and analysis This study is a multicentre, two-by-two factorial randomised controlled trial evaluating the efficacy of immunonutrition supplementation and oral chlorhexidine decontamination. A total of 592 patients aged 65 years and older who are scheduled for elective non-cardiac surgeries in seven tertiary hospitals in China will be recruited. Patients will be excluded if they have contraindications to the intervention. Patients will be randomised into four groups in a 1:1:1:1 ratio (oral decontamination vs routine oral care, immunonutrition supplementation vs routine nutrition advice). The primary outcome is the incidence of PPCs within 7 days after surgery. The secondary outcomes are the incidence of postoperative pneumonia, infectious complications, Comprehensive Complication Index, postoperative functional recovery, length of hospital stay and hospital expenses. Intention to treat principles will be applied to all outcomes. Descriptive analysis will be used to compare patients’ baseline characteristics. Logistic regression will be used to compare the incidence of PPCs within 7 days after surgery between different groups.
Ethics and dissemination The study protocol has been approved by the Research Ethics Committee of Peking Union Medical College Hospital (I-23PJ953). All participants will provide written informed consent. Study results will be published in peer-reviewed journals and presented at academic conferences.
Trial registration number NCT05971810.
- SURGERY
- ANAESTHETICS
- Aged
- Nutritional support
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Using a multicentre, randomised controlled trial design, this study will provide high-quality evidence on the efficacy of preoperative immunonutrition supplement and oral decontamination.
The two-by-two factorial design allows comparison of two perioperative optimisation plans to efficiently prevent postoperative pulmonary complications in elderly surgical patients.
The factorial design also allows exploration of statistical interactions between the two interventions; however, significant interactions might reduce statistical power for affirmative conclusions.
Patients will not be blinded to the interventions; therefore, performance bias might be introduced.
Introduction
Elderly patients are associated with higher risks of postoperative pulmonary complications (PPCs), especially pneumonia,1 bringing significant medical and economic burden.2 Senior age, preoperative respiratory diseases, long-term smoking history, recent respiratory infection, preoperative anaemia and longer surgical duration have been considered as contributing factors to the higher risk of PPCs in the general surgical population.3
Decline of respiratory function and impairment of upper respiratory tract protection mechanisms associated with ageing may become particularly relevant in the perioperative period, when anaesthesia and surgical factors may impose additional burden on elderly patients. Prehabilitation programmes targeting respiratory strength have been demonstrated to be effective in reducing pulmonary complications among patients undergoing pulmonary procedures,4 5 suggesting weakness of respiratory muscles as a possible contributor to PPCs. Nutritional support may compensate for the catabolic status of surgical patients, helping reinforce respiratory function and develop an effective response to infection. In addition, immunomodulating nutrition has positive clinical effects by altering inflammatory response and enhancing the host defence system.6 However, the effects of immunonutrition support among elderly surgical patients are not supported by high-grade clinical evidence.
On the other hand, during the perioperative period, worse oral hygiene, higher risk of aspiration associated with sedation or intubation, and reduced mucociliary function due to mechanical ventilation or medications all add to the risk of disrupted lung microbiome and further lead to pneumonia. Microbes from the oral cavities have been suggested as the primary driver of the lung microbiome.7 Endotracheal tubes have also been found to be colonised with microbes from the oral cavity, subsequently forming a biofilm and promoting ventilator-associated pneumonia.8 The effects of pneumonia prevention bundles including chlorhexidine oral decontamination have been demonstrated among intensive care unit patients and cardiac surgery patients.9 Theoretically, oral decontamination may prevent pulmonary complications by reducing micro-organism translocation from the oral/gastrointestinal tract to the pulmonary microbiome. However, among non-cardiac surgical patients, who do not routinely require prolonged mechanical ventilation, the effect of perioperative chlorhexidine is less conclusive.10 11
In this study, we postulate that reduced pulmonary functional reserve associated with ageing and higher risk of lung microbiome homeostasis disruption are two possible mechanisms leading to higher risk of PPCs in elderly surgical patients. We hypothesise that perioperative oral chlorhexidine decontamination and perioperative immunonutrition supplementation (POINT) are effective in preventing PPCs among elderly non-cardiac surgical patients.
Methods and analysis
Study design
This study is a multicentre, two-by-two factorial randomised controlled trial. Eligible patients scheduled for elective non-cardiac surgery at Peking Union Medical College Hospital (PUMCH), Fujian Provincial Hospital, The Second Hospital of Hebei Medical University, Peking University International Hospital, Ningbo No 2 Hospital, Shenzhen Qianhai Shekou Free Trade Zone Hospital and Guizhou Provincial People’s Hospital will be enrolled. This protocol is reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials guidance (online supplemental appedix 1), and has been approved by the Research Ethics Committee of the PUMCH (reference number I-23PJ953) and by each centre. The study protocol has been prospectively registered at ClinicalTrials.gov (NCT05971810).
Supplemental material
Participants and eligibility criteria
Patients who meet all the following criteria will be considered for inclusion: (1) age ≥65 years; (2) undergoing major non-cardiac surgery (expected duration of surgery >2 hours); (3) scheduled for general anaesthesia and endotracheal intubation; (4) with American Society of Anesthesiologists (ASA) physical status classification I–IV; (5) at intermediate to high risk of respiratory complications, defined as ARISCAT (Assess Respiratory Risk in Surgical Patients in Catalonia)3 score ≥26; and (6) provided informed consent. Patients who meet any of the following criteria will be excluded: (1) emergency surgery; (2) preoperative pneumonia; (3) allergy to chlorhexidine; (4) severe hepatic dysfunction (Child-Pugh class C), severe renal dysfunction (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2), incapable of oral feeding, with autoimmune diseases, taking immunosuppressant or immunoregulation medications, or with other contraindications to immunonutrition supplementation; and (5) expected intervention of immunonutrition <3 days.
Patients are able to withdraw their consent on personal request or when the surgery is cancelled. Patients will be assessed at the preoperative anaesthesia clinic, and those eligible will be enrolled after providing written informed consent (online supplemental appendix 2).
Supplemental material
Comparisons
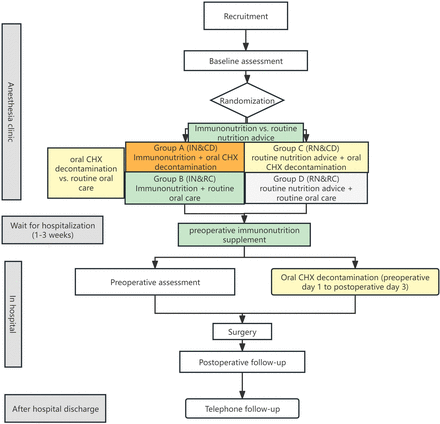
There will be two interventions following a factorial design in this study. Participants will be randomised to receive either oral chlorhexidine rinse or routine oral care, and to receive immunonutrition supplementation or standard nutrition advice. Apart from the intended comparisons, all patients will follow standard clinical practice in other aspects, and any concomitant care as part of routine care is permitted (figure 1).
Study flow diagram. CHX, chlorhexidine.
Comparison 1: oral chlorhexidine decontamination versus routine oral care
Patients in the control group will receive routine oral care, which is routine toothbrushing two times per day. Patients in the intervention group will receive oral decontamination using 0.12% chlorhexidine oral rinse two times per day from the day before surgery to postoperative day (POD) 3.
Comparison 2: immunonutrition supplementation versus standard nutrition advice
Patients in the control group will be advised to follow standard nutrition advice with a total intake of 30 kcal/kg/day and a protein intake of 1.2 g/kg/day. Apart from nutrition advice, patients in the intervention group will also receive additional immunonutrition supplementation from the day of allocation at the preoperative anaesthesia clinic to the day before surgery (usually 1–3 weeks), which is oral intake of ORAL IMPACT (containing L-arginine, omega-3 fatty acids and nucleotides, which are major immunonutrition components) with two servings per day. In consideration of gastrointestinal tolerance, immunonutrition supplement will be given in a gradually built-up manner. A twice-a-week telephone follow-up will be conducted and a diet diary will be recorded to improve adherence.
Intraoperative management
Anaesthesia management
All patients are recommended to be induced by propofol, fentanyl and rocuronium. Total intravenous anaesthesia with propofol target-controlled infusion of plasma concentration 2.5–5 μg/mL to maintain bispectral index of 40–60 will be advised. The attending anaesthesiologists are allowed to individualise anaesthesia management according to the patient’s medical condition. For airway management, oral endotracheal intubation and protective ventilation strategy will be performed. The reference mechanical ventilation mode is volume control mode or volume-guaranteed pressure control mode, with tidal volume of 6–8 mL/kg according to predicted body weight, fraction of inhaled oxygen (FiO2) 50%, positive end-expiratory pressure (PEEP) 4–10 cmH2O and plateau pressure (Pplat) <30 cmH2O. Recruitment manoeuvre after anaesthesia induction and shortly before extubation will be performed. For intraoperative fluid management, restricted fluid infusion strategy will be adopted with adequate infusion of balanced crystalloids to compensate for basic physiological requirements. Blood products or colloids can be administered for resuscitation in the event of blood loss and other fluid loss. Surgical type, intraoperative surgical position, placement of nasogastric tube, combination of intrathecal or regional anaesthesia, and use of antibiotics, opioids, neuromuscular blockers and reversal medications will be recorded as potential confounding factors.
Outcomes
The primary outcome is PPCs within 7 postoperative days. The secondary outcomes include (1) PPCs within 30 postoperative days; (2) postoperative pneumonia and infectious complications within 7 days and 30 days after surgery; (3) Comprehensive Complication Index (CCI) within 7 days and 30 days after surgery12 13; and (4) length of hospital stay, hospital expenses, score on the 15-Item Quality of Recovery Scale (QoR-15) at 1–3 days and 7 days after surgery, score on the WHO Disability Assessment Schedule (WHODAS 2.0) at 30 days after surgery, and functional recovery including day of self-urination, day of self-defecation and day of off-bed activity.
PPCs include pneumonia, atelectasis, hypoxaemia, respiratory failure, acute respiratory distress syndrome, aspiration pneumonia, prolonged mechanical ventilation, unplanned reintubation, pleural effusion, pneumothorax and bronchospasm. Detailed definitions of each PPC are provided in online supplemental appendix 3.
Supplemental material
Sample size
The incidence of PPCs reported in previous studies varied from 5.5% to 33.4%.14–17 Previous studies on perioperative oral care revealed a 30%–60% reduction of pneumonia10 and a 20%–80% reduction of infection complications by immunonutrition supplementation.18 19 Considering the patients in this study are elderly who are at higher risk, assuming a bilateral statistical risk at 5% and 10% for type I and type II errors and a 20% PPC in the control group, 532 patients will be required to detect a 50% reduction in PPC risk. Considering a possible attrition of 10%, we plan to enrol 148 patients for each of the four groups, with a total of 592 patients.
Recruitment, randomisation and treatment allocation
Patients will be referred to our preoperative anaesthesia clinic by the corresponding surgeons after being scheduled for surgery (usually 1–3 weeks prior to the surgery). Patients will be assessed for eligibility at the preoperative anaesthesia clinics by the researchers. After providing written informed consent, patients who are recruited will be further assessed in terms of baseline information and then allocated into different study groups. This study is currently recruiting participants. We plan to finish recruitment over a 24-month recruitment period, starting from August 2023 to August 2025. The study is expected to be completed in September 2025.
Enrolled patients will be randomised into four groups: group A (immunonutrition and oral chlorhexidine decontamination), group B (immunonutrition and routine oral care), group C (routine nutrition advice and oral chlorhexidine decontamination) and group D (routine nutrition advice and routine oral care). Participants will be randomly assigned to these four groups in a 1:1:1:1 ratio. The random allocation sequence will be generated through a central computer. Block randomisation with a block size of 8 will be used. Randomisation will be accessed via a central secure system and recorded by a third party to achieve adequate allocation concealment.
Blinding
The surgeons, anaesthesiologists, outcome assessors and data analysts are unaware of the allocation. Patients are not blinded in this study.
Data collection and management
Data collection and recording will follow standard case report forms. Sociodemographic information, basic medical history and baseline functional status will be collected by trained study personnel. Baseline assessment will be completed during the preoperative anaesthesia clinic after recruitment. Preoperative height, weight, body mass index, frailty assessment (Fried phenotype), grip strength, Short Physical Performance Battery (SPPB), Nutritional Risk Screening 2002, Mini-Mental State Exam, peripheral oxygen saturation breathing room air and comorbidities including those known to increase risk of aspiration (history of gastro-oesophageal reflux, functional or mechanical gastrointestinal obstruction, previous oesophageal surgery, head injury, active emesis or active upper gastrointestinal bleeding) will be assessed and recorded.
On admission to the hospital for the scheduled surgery, patients will be reassessed in terms of frailty status, nutritional status and physical function. Ultrasound assessment of the masseter and temporalis muscles will be performed preoperatively and on POD 3 to assist in the evaluation of muscle status. Revised Oral Assessment Guide and water drinking test will be assessed preoperatively for oral health and swallowing function.
During the hospital stay, patients will be visited daily for postoperative follow-up, especially during the first week after surgery. After hospital discharge, all patients will be telephoned on POD 30 for follow-up. Electronic medical records will also supplement the follow-up. Pulmonary ultrasound will be performed to calculate quantitative lung ultrasound score20 before surgery, within 24 hours after surgery and on POD 3. Patients’ day of self-urination, day of self-defecation and day of off-bed activity will be recorded. Postoperative functional tests, including SPPB and grip strength, will be reassessed on POD 7 or the day before discharge. QoR-15 will be assessed preoperatively, on POD 1–3 and POD 7, or the day before hospital discharge, whichever comes first. WHODAS 2.0 will be assessed preoperatively and on POD 30.
Peripheral blood sample will be taken to test for inflammatory and immune panel, fasting blood glucose level and insulin level as exploratory analyses in certain participating centres.
An electronic data capture system will be used in a privacy legislation-compliant manner. Patients’ information will be stored in standard format, and paper case report forms will be stored in a secured cabinet.
Data analysis
Intention to treat principles will be applied to all outcomes. Descriptive analysis will be used to compare patients’ baseline characteristics. The primary outcome will be analysed using logistic regression comparing the incidence of PPCs within 7 days after surgery between different groups, and the relative risk with 95% CI will be calculated as effect size. Both crude and adjusted coefficients will be reported. A similar strategy will be used in analysing dichotomous secondary outcomes. A t-test will be used to compare postoperative CCI between groups, and the mean difference with 95% CI will be reported as an effect size measure. The length of hospital stay will be analysed using Cox regression model, where time to discharge is regressed against treatment allocation, with HR along with 95% CI as the effect size measure and in-hospital mortality as a competing risk. Hospital expenses will be analysed using the Mann-Whitney U test, and the median difference with 95% CI estimated by the Hodges-Lehmann method will be reported as the effect size measure. QoR-15 will be analysed using repeated measures analysis of variance. A subgroup analysis will be performed based on age, sex, ASA classification, preoperative nutritional status, surgical type and condition of oral health.
With regard to outcomes, two intervention comparisons, oral decontamination versus routine oral care, and immunonutrition supplementation versus standard nutrition advice, will be conducted separately. For each comparison, a two-sided p<0.05 will be considered statistically significant. Based on their theoretical mechanisms to prevent PPCs, the interaction effect between oral decontamination and immunonutrition supplementation regarding the primary outcome will be tested as an exploratory analysis, by using a log-binomial model including both the main and the interaction effect. We will not adjust type I error among the secondary outcomes and the subgroup analysis. Hence, relevant findings will also be regarded as exploratory results.
Monitoring
Data and safety monitoring will be performed by the principal investigator, coinvestigators and biostatisticians. Blinded interim analyses are not planned in this study. All adverse events during data collection and intervention will be documented. If any serious adverse event related to the protocol occurs, the intervention will be interrupted if necessary and the event will be reported to the local research ethics committee as soon as possible.
Patient and public involvement
None.
Ethics and dissemination
This study protocol (protocol version 2.0; date: 24 May 2023) has been approved by the Research Ethics Committee of the PUMCH (reference number I-23PJ953) and by each participating centre. All participants will be given oral and written information about the study and will provide written informed consent.
Any significant modifications to the protocol, including changes to the study population, sample size, study design or study procedures, will require a formal amendment approved by the local research ethics committee. The study has been registered prospectively at ClinicalTrials.gov (NCT05971810; 2 August 2023). Findings of this research will be disseminated via peer-reviewed publication and scientific conferences.
Ethics statements
Patient consent for publication
Acknowledgments
We thank all the staff at each participating centre for their contributions to the study.
Footnotes
Contributors JY, LC, QZ, LX, JF and YH were involved in the conception and design of the study. JY, LC and YZ drafted the manuscript. QZ, MY, XZ, CL, LH, WW, LY, GF, JC and JZ are participating in the implementation of the study. YZ was involved in the study methodology and analysis plan. YH is the principal investigator and guarantor of this study. All authors read and approved the final protocol.
Funding This study is funded by the Peking Union Medical College Hospital National High Level Hospital Clinical Research Funding (2022-PUMCH-B-006). This funding source had no role in the study design, execution, analysis, interpretation or publication decisions.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.