Article Text
Abstract
Introduction The current pharmacological management of type 2 diabetes mellitus (T2DM) faces challenges such as low rates of optimal glycaemic control, high incidences of adverse drug reactions and suboptimal treatment compliance. Pueraria lobata radix (PLR), a medicinal and edible herb, has shown hypoglycaemic effects in animal models. However, existing clinical studies have only assessed the hypoglycaemic effect of PLR-containing herb formulas or PLR extract preparations. The aim of this study is to investigate the efficacy and safety of using PLR solely as an adjuvant therapy for T2DM.
Methods and analysis This study is a multicentre, randomised, double-blind, placebo-controlled trial. 200 patients with T2DM will be randomly allocated to either the PLR group or the placebo group for a consecutive 12-week intervention. Regular visits will be conducted at weeks 4, 8 and 12, following the initiation of the study to evaluate the efficacy and safety of PLR. The primary outcome is the change in haemoglobin A1c (HbA1c) from baseline at week 12. Secondary outcomes include changes in HbA1c from baseline at weeks 4 and 8; the HbA1c response rate (< 7%), changes in fasting blood glucose, 2-hour blood glucose, fasting C-peptide, body mass index, severity of diabetes symptoms, quality of life from baseline at weeks 4, 8 and 12; and changes in blood lipid indicators at week 12. Safety outcomes include the incidences of total adverse events (AEs), serious AEs and PLR-related AEs.
Ethics and dissemination The protocol has been approved by the Ethics Committees of the First Affiliated Hospital of Nanchang University (approval number: IIT[2024]LLS No.303) and the Affiliated Hospital of Jiangxi University of Chinese Medicine (approval number: JZFYLL2024006200087). We will disseminate the study findings through publications in peer-reviewed journals and conference presentations.
Trial registration number ClinicalTrials.gov NCT06494683.
- Randomized Controlled Trial
- Diabetes Mellitus, Type 2
- Safety
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This trial will employ rigorous methodological safeguards, including multicentre recruitment, block randomisation, allocation concealment, double blinding and placebo control, to balance both known and unknown confounders and minimise the placebo effect.
Multiple compliance-guarantee measures, blinded outcome assessments and reasonable statistical analysis plans will improve trial quality and yield accurate evaluation results.
A sample size of 200 participants will be sufficient to ensure the statistical power and the credibility of the prespecified primary and subgroup analyses.
A potential limitation of the study is that patients may not fully adhere to the 12-week Pueraria lobata radix or placebo regimen.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder with the highest prevalence.1 Data released by the International Diabetes Federation in 2021 indicated that the number of patients with T2DM among the population aged 20−79 years has soared to 537 million, accounting for 10.5% of the total global population, and it is predicted that the number will escalate to 783 million by 2045.2 The chronic hyperglycaemic state leads to multiple complications, such as cardiovascular diseases, strokes, blindness, renal failure and foot ulcers.3 4 These serious consequences are among the chief causes of premature mortality and reduced life expectancy in humans.5 6 Furthermore, the medical expenditures associated with T2DM impose an immense socioeconomic burden—the total global medical expenses for T2DM in 2021 amount to US$966 billion, representing a 27.1% increase from 2019.7 This figure, if considered a country’s GDP, could secure 18th rank globally.8
T2DM is conventionally managed through oral hypoglycaemic drugs and insulin.9 These drugs can increase insulin levels, alleviate insulin resistance and achieve the goal of lowering blood glucose. However, a significant proportion of patients still fail to attain optimal blood glucose control.10 11 The principal cause of this problem is the limited hypoglycaemic ability of existing oral hypoglycaemic drugs.12 13 For example, a network meta-analysis of multiple hypoglycaemic drugs revealed that, compared with that of placebo, the reduction in haemoglobin A1c (HbA1c) ranged from −1.48% for the highest semaglutide to only −0.50% for the lowest dipeptidyl peptidase-4 inhibitors.14 Secondary failure is also a challenging issue. For example, 42% of T2DM patients who received metformin, a first-line monotherapy drug, experienced secondary failure during the average follow-up period of 27.6 months, corresponding to a failure rate of 17% per year.15 The combination of multiple hypoglycaemic drugs is a way to solve these issues; however, it considerably increases adverse drug reactions, such as hypoglycaemia16 and gastrointestinal symptoms.17 Moreover, compliance is a crucial factor affecting the effectiveness of hypoglycaemic drugs. A survey has revealed that the proportion of patients with good compliance with hypoglycaemic drugs is less than 40%.18 Adverse drug reactions and complex treatment regimens (combination therapy or the requirement for injection) are the primary reasons for poor patient compliance.19–21 Therefore, it is imperative to explore complementary therapies that can enhance efficacy, safety and long-term compliance for the management of T2DM.
Pueraria lobata radix (PLR; Chinese name: Gegen) is the dry root of the leguminous plant P. lobata (Willd.) Ohwi. In East Asian countries, particularly in China, PLR has been used as an ingredient in traditional herbal prescriptions for T2DM. For example, Gegen Qinlian decoction, composed of PLR, Scutellaria baicalensis, Coptis chinensis and Glycyrrhiza uralensis, has been found to significantly reduce the level of blood glucose and increase the level of serum insulin.22 23 Isoflavones in PLR, such as daidzein, genistein and puerarin, have been shown to have hypoglycaemic activity. The mechanism of hypoglycaemic action may involve multiple signalling pathways, such as the PI3K-Akt, PPAR and HIF-1 pathways.24–26 Recent studies have also found that the polysaccharides contained in PLR also have hypoglycaemic effects, which improve insulin resistance in mice by regulating the PPAR signalling pathway.27
In China, PLR is a medicinal and edible herb, as stipulated by regulations; that is, based on traditional consumption experience, it is considered to have food-level safety and can be made directly into foods or can serve as a food ingredient. The safety evaluation of standardised PLR extracts also reached a conclusion of being favourable for safety.28 These facts provide a prerequisite for its application in the dietotherapy of T2DM. However, to date, all clinical studies regarding the hypoglycaemic effect of PLR have focused on PLR-containing herbal formulas or preparations of PLR extracts, and there is no evidence from randomised controlled trials (RCTs) for assessing PLR alone for adjunctive hypoglycaemic management. Therefore, we plan to conduct an RCT to assess the efficacy and safety of PLR as an adjuvant therapy in the management of T2DM.
Methods and analysis
Study design
This study will be a multicentre, randomised, parallel-group, double-blind, placebo-controlled trial. This protocol has been registered at clinicaltrials.gov (ID: NCT06494683). Participants will be recruited at the First Affiliated Hospital of Nanchang University and the Affiliated Hospital of Jiangxi University of Chinese Medicine. Eligible patients with T2DM will be randomly assigned to either the PLR group or the placebo group. The reporting of this trial protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement.29 The study process is shown in figure 1.
Flowchart of the study process.
Eligibility criteria
Participants will be recruited during physicians’ regularly scheduled outpatient clinics, with advertisements by online and offline posters. Potential candidates will be evaluated by clinicians with professional qualifications in endocrinology to determine whether they meet the following inclusion criteria to participate in this trial:
Diagnosed with T2DM according to the criteria of the American Diabetes Association, that is, fasting blood glucose (FBG) ≥126 mg/dL (7.0 mmol/L) or blood glucose ≥200 mg/dL (11.1 mmol/L) 2 hours after oral administration of 75 g glucose or HbA1c ≥6.5% (48 mmol/mol).30
Treatment-naïve patients (including newly diagnosed or previously diagnosed but untreated) or those who have been receiving regular hypoglycaemic drugs for at least 3 months (both oral hypoglycaemic drugs and insulin are allowed, regardless of the type and dose).
Blood glucose has not been effectively controlled for the past 3 months, defined as an HbA1c between 6.5% and 10.5%.
Aged between 18 years and 80 years.
Agree to the requirements of dietary control during the study period (see paragraph 3 of the ‘Interventions and cointerventions’ section).
Voluntarily participate and sign the informed consent form.
Patients with the following conditions will not be eligible to participate:
Type 1 diabetes mellitus, gestational diabetes mellitus or other special types of diabetes mellitus.
Experienced acute complications of T2DM, such as ketoacidosis, hyperosmolar coma, lactic acidosis and acute hypoglycaemia.
Irregular hypoglycaemic treatment patterns, such as intensive therapy phases or inconsistent daily medication types/dosages.
Pregnant or lactating women or women planning to become pregnant.
A history of allergy to PLR.
Complicated with severe dysfunction of important organs, such as the heart, liver and kidney; malignant tumours; or severe mental disorders.
Anticipated poor compliance, such as remote residence from the study site, frequent work-related travel and a clinician-determined history of medication non-adherence.
Currently participating in other clinical trials.
Interventions and cointerventions
Participants in the PLR group and the placebo group will receive PLR and placebo treatment, respectively, once daily for 12 weeks, with a dosage of 15 g per dose. This dosage adopts the upper limit of the Chinese Pharmacopoeia-recommended daily range (10–15 g/d, established by the Chinese National Pharmacopoeia Committee based on traditional clinical experience) to prioritise safety while maximising therapeutic efficacy.31 The 12-week intervention period is determined by referencing previous RCTs of herbal formulas containing PLR to align with the anticipated efficacy onset period of PLR,23 while also ensuring sufficient time for HbA1c to accurately reflect glycaemic changes.32 PLR and placebo will be made into granules that can be dissolved in warm water for convenient administration. To produce granules, PLR decoction pieces will be fully soaked and then decocted with water three times, and all the decocted liquids will be combined, concentrated and dried to form granules. The placebo granules will be made by blending lactose, caramel colour, sunset yellow and PLR essence. There will be no or almost no difference in colour, smell, taste, appearance or packaging between the PLR and placebo preparations.
During the study period, untreated participants will receive hypoglycaemic drugs following standard treatment protocols, with the initial type and dosage of hypoglycaemic drugs maintained. For participants already on regular hypoglycaemic drug regimens, the drug type and dosage will be sustained throughout the study period. When clinicians determine that a participant’s blood glucose levels become uncontrolled (either too high or too low), rescue treatment will be implemented. A high blood glucose level is defined as an FBG level exceeding 270 mg/dL (15.0 mmol/L) during weeks 1–6 or exceeding 239 mg/dL (13.3 mmol/L) during weeks 7–12, according to the Food and Drug Administration’s recommendations.33 Rescue treatment for high blood glucose levels includes upward titration of current antidiabetic medications to their maximum as per prescribing information. If blood glucose levels remain unacceptably high, additional hypoglycaemic drugs will be introduced, prioritising metformin (to be gradually titrated to a maximum dose of 2 g/d) followed by glimepiride (to be gradually titrated to a maximum dose of 6 mg/d). A low blood glucose level is defined as an FBG level <70 mg/dL (3.9 mmol/L). In such cases, the original hypoglycaemic drug dosage will be reduced to maintain the FBG level above 70 mg/dL, with complete discontinuation if necessary. For acute hypoglycaemic episodes, rescue treatment will involve the administration of 15–20 g of rapid-acting carbohydrates (eg, sugary beverages, candy or honey) to resolve symptoms. All rescue treatments will be documented and considered in per-protocol population analysis.
All participants will be required to regulate their diet, including restricting the intake of monosaccharides, daily fat intake and total calories based on body weight and to adhere to a diet pattern of small and frequent meals, a light diet and punctual eating. Other herbal remedies will be prohibited. Treatment of comorbidities, such as hypertension and dyslipidaemia, as well as adverse reactions during the study period, is permitted. In the event of a violation of the intervention or co-intervention protocol, participants should truthfully report the type and dosage of the violation and make detailed records, while subsequent interventions and visits will proceed.
Randomisation and blinding
An independent randomisation centre will be established to generate and manage random sequences. The ‘blockrand’ function in R V.4.1.0 (Ross Ihaka, Robert Gentlemen, New Zealand) will be used to generate random sequences with mixed block lengths of 4, 6 or 8, featuring a 1:1 allocation ratio between the PLR group and the placebo group. One random sequence with a fixed seed will be generated for each research centre. Sealed packaging will be adopted to conceal random sequences. First, the central pharmacy will arrange the PLR and placebo samples according to the random sequences and then affix sequential numbers on the packaging. When patients participate in the trial, the samples will be distributed according to the enrolment sequence. Therefore, neither the clinicians nor the participants can be aware of the grouping results. Before the completion of data analysis, all participants, clinicians, outcome evaluators and data analysts will be blinded to the randomised grouping. In the case of serious adverse events (AEs) during the study, emergency unblinding is allowed to provide necessary information for treatment.
Outcomes
Efficacy outcomes
The primary outcome for efficacy assessment is the change in HbA1c at week 12 from baseline. Secondary efficacy outcomes include: (1) changes in HbA1c at weeks 4 and 8 from the baseline; (2) the HbA1c response rate at weeks 4, 8 and 12, defined as HbA1c <7.0%; (3) changes in other blood glucose indicators at weeks 4, 8 and 12, including FBG, 2-hour postprandial blood glucose and fasting C-peptide; (4) changes in body mass index, systolic blood pressure and diastolic blood pressure at weeks 4, 8 and 12; (5) changes in levels of blood lipid indicators at week 12, including total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol; (6) changes in the severity of diabetic symptoms at weeks 4, 8 and 12 evaluated by the score of the Diabetic Symptom Severity Grading Scale in the Clinical Research Guiding Principles for New Drugs of Chinese Medicine;34 the scale contains 24 items and assesses the main symptoms of diabetes, such as excessive thirst and desire to drink, excessive eating and easy to be hungry, frequent urination and frequent nocturia, with each item scoring 0–3 (total, 0–72) and a higher score indicating more severe symptoms; (7) changes in the quality of life of patients at weeks 4, 8 and 12 evaluated by the score of the Diabetes-Specific Quality of Life Scale;35 the scale contains 27 items and assesses the impact of diabetes on the quality of life of patients in four dimensions of physiology, psychology, society and treatment, with each item scoring 0–4 (total, 0–108) and a higher score indicating a poorer quality of life; and (8) changes in the dose of hypoglycaemic drugs at weeks 4, 8 and 12 from the baseline.
Safety outcomes
Safety outcomes include the incidences of any AEs, serious AEs and PLR-related AEs within 12 weeks. An AE is defined as any adverse symptom or event that is not related to the natural progression of the patient’s original disease. The data sources for safety evaluation include tests of blood, urine and stool routine; stool occult blood; FBG; liver function (including alanine aminotransferase, aspartate aminotransferase and total bilirubin); and kidney function (including serum creatinine, urea nitrogen and uric acid) and ECG examinations. Among these examinations, if the baseline level is normal and the indicator level at follow-up exceeds twice the upper limit of the normal reference value or is reported as qualitatively abnormal, it will be considered an AE. The incidences of symptomatic hypoglycaemia and gastrointestinal symptoms will be monitored with emphasis. Symptomatic hypoglycaemia is defined as a blood glucose level <3.9 mmol/L accompanied by hypoglycaemic symptoms, such as palpitations, sweating, hunger, tremors and anxiety. We will document the time, location, course of disease, clinical manifestations, management and outcome of each AE. The correlation between AEs and PLR treatment will be comprehensively evaluated by clinicians based on the following factors: (1) whether there is a reasonable temporal relationship between the occurrence of the AE and the administration of PLR; (2) whether the AE can be explained by other factors; (3) whether the AE has been reported in the previous literature or has reasonable biological mechanisms; (4) whether the AE disappears or alleviates after the discontinuation or reduction of PLR; and (5) whether the same AE recurs after readministering PLR. A serious AE is defined as an AE that leads to hospitalisation or a prolonged hospital stay, affects work ability, endangers life or causes disability, congenital malformations or death. Any serious AEs will be immediately reported to the hospital ethics committee.
Schedule of visits
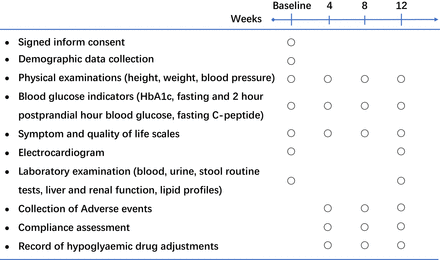
Study visits will be conducted at baseline and at weeks 4, 8 and 12. During the baseline visit, we will collect demographic data, disease history and medication history; examine baseline vital signs, blood glucose indicators, blood lipid indicators and safety indicators; and measure baseline scores on the severity of symptoms and quality of life scales. At each subsequent follow-up visit, we will perform examinations or measurements of vital signs, blood glucose indicators and symptom and quality of life scales and record compliance with interventions, newly emerged diseases or disease progression, the dose of hypoglycaemic drugs and AEs. At the last follow-up, we will additionally examine lipid indicators and laboratory indicators related to safety. The time window for each follow-up visit is ±3 days from the scheduled visit date. If a patient fails to complete the follow-up within the scheduled time for any reason, we will consider this visit as lost to follow-up and no longer collect the data from this visit. The detailed follow-up arrangement is shown in figure 2.
Schedule of the study visits.
Quality control and data management
A steering committee composed of endocrinology experts, herbal medicine experts, statistics experts, ethics experts and the director of the research hospital will be established. This committee will be responsible for formulating and reviewing the study protocol, establishing standard operating procedures and organising and supervising the quality of implementation. This trial has no interim analysis plan, but the steering committee can decide to conduct an interim analysis depending on the progress of the trial and determine whether to prematurely terminate the trial based on the results of the interim analysis, such as when the interventions are unlikely to achieve any significant efficacy or when there are major safety issues. Each research hospital will be equipped with two full-time clinical research coordinators, who are responsible for cooperating with clinicians to conduct research visits. All researchers will fully study the study protocol and undergo training on standard operating procedures. Every patient participating in the trial will receive one-on-one reception, including disease consultation, the completion of case report forms, the collection of biological samples and the distribution of PLR or placebo samples. To increase compliance, we will first obtain thorough informed consent for each participant, including randomisation grouping, the potential benefits and side effects of each group and the responsibilities for participating in the study. The trust of patients in clinicians and their interest in the research are the core factors to guarantee compliance. Second, clinical research coordinators will actively remind patients when the planned follow-up date is approaching and respond to patients inquiries regarding health or the study protocol at any time. In addition, we will provide participants with free PLR and placebo samples and free research-related examinations, as well as transportation subsidies for each return visit (100¥ per person per visit). Patients in the placebo group will be compensated with the same amount of PLR samples as those in the experimental group after the completion of the trial. To verify the compliance of the self-reported dose of the trial samples, patients will be required to return the empty packaging bags of the consumed samples and the packaging bags of the remaining samples for obtaining free samples in the next month and transportation subsidies.
An independent data monitoring committee will be established to manage the research data and regularly inspect the quality of data collection. The original data will be recorded in paper case report forms and stored in locked boxes at each research centre. After the completion of the data collection, the data of the paper case report forms will be re-entered into the electronic database constructed by Microsoft Access by two independent staff members, with cross-verification to ensure accuracy. All patient information will be kept strictly confidential.
Sample size calculation
No prior clinical studies have directly compared PLR with a placebo in the treatment of T2DM. However, a meta-analysis of 17 RCTs investigating Gegen Qinlian decoction (an herbal formulation primarily containing PLR) demonstrated a −0.65% greater reduction in HbA1c compared with control groups (exercise and diet control) after 8–12 months of intervention.23 Drawing from these findings, combined with our empirical clinical observations of PLR therapeutic effects and expert consensus, we anticipate the mean difference (δ) in the reduction of the primary outcome (ie, the change in HbA1c levels at week 12) between the PLR group and the placebo group to be 0.50% and the common standard deviation (σ) between the groups to be 0.75%. With the assumed type I error probability of 0.05 and type II error probability of 0.20, along with a potential 20% loss to follow-up, a sample size calculation formula of the superiority design indicates that at least 47 cases are needed for each group. To increase the credibility of subgroup analyses, we plan to expand the sample size to 100 cases for each group within the limits of funding support, resulting in a total of 200 cases.
Statistical analysis
Descriptive statistics will be conducted for baseline characteristics, where continuous variables will be presented as means and SD, and categorical variables will be presented as frequencies and percentages. The analysis of efficacy outcomes will use the full analysis set based on the modified intention-to-treat principle, encompassing the population that has received at least one dose of the PLR or placebo after randomisation and has outcome data of at least one follow-up. The estimation of intergroup differences in continuous outcomes will employ a repeated-measures mixed effects model, in which age (continuous), body mass index (continuous), baseline HbA1c (continuous) and use of insulin (binary) are fixed-effect covariates, and research centre, time and interaction of time and group are random-effect covariates. The effect size on continuous outcomes will be least-square mean differences and 95% CIs. The estimation of intergroup differences in binary outcomes will employ a repeated-measures generalised linear mixed model with the logit link, with the same covariates. The effect size on binary outcomes will be ORs and 95% CIs. Missing values in outcomes will be imputed via the multiple imputation method based on the regression model, where covariates include age, body mass index, baseline HbA1c, use of insulin and the research centre. 100 imputed datasets will be generated and used to fit the statistical models, and the results will be averaged as the final effect estimate. The analysis of safety outcomes will be based on the safety set, defined as the population that has received at least one dose of PLR or placebo.
To verify the robustness of the results of the efficacy analysis, the following sensitivity analyses will be conducted: (1) using the per-protocol set, which is defined as the population that has consumed more than 80% of the PLR or placebo samples, has not violated the intervention and co-intervention requirements, has not adjusted the dose of hypoglycaemic drugs or added new hypoglycaemic drugs compared with the baseline prescription, has no major violations of the dietary control requirements, has not been unblinded and has completed the week 12 visit; (2) abstaining from imputing missing values; and (3) adjusting any additional unbalanced baseline characteristics in the statistical models.
To provide evidence for precise treatment, we will conduct subgroup analyses stratified by the following factors for the primary outcome: (1) age (<65 years vs ≥65 years); considering the potential impact of age on drug absorption and metabolism ability, it is anticipated that participants aged <65 years will achieve superior efficacy; (2) baseline body mass index (<23.0 kg/m2 vs ≥23.0 kg/m2); considering the potential effects of PLR on inhibiting abnormal leptin receptors, it is anticipated that participants with a body mass index ≥23.0 kg/m2 will achieve superior efficacy; (3) baseline HbA1c level (<9% vs ≥9%); considering the impact of baseline HbA1c control on the absolute level of HbA1c reduction, it is anticipated that participants with a baseline HbA1c ≥9% will achieve superior efficacy; and 4) the use of insulin (yes vs no); considering the potentially stronger masking effect of insulin on the efficacy of PLR, it is anticipated that participants not receiving insulin will achieve superior efficacy.
All analyses will not adjust the significance boundary; that is, a p value <0.05 will be considered statistically significant. All the statistical analyses will be performed in SAS V.9.4 (SAS Institute, Cary, North Carolina, USA).
Patient and public involvement
The trial design has not yet involved patients or the public.
Discussion
There are more than 20 species of plants belonging to the genus Pueraria worldwide, primarily distributed in subtropical and temperate regions.36 China constitutes the distribution centre of the genus Pueraria, comprising approximately 10 species. Among them, P.lobata (Willd.) Ohwi and P.montana var. thomsonii are the species endowed with the most abundant resources and the widest cultivation scope, and their roots are also the types legally defined as medicinal and edible herbs in China.37 38 Compared with that in P.thomsonii radix, the total flavonoid content in PLR, particularly puerarin, is significantly greater. Therefore, PLR holds greater medicinal value and is more prevalently employed in herbal prescriptions than P.thomsonii radix.39 As a result, we chose PLR as the evaluation object in this trial.
This study will be the first RCT to evaluate the efficacy and safety of PLR as an adjunctive treatment for T2DM. Previous clinical trials concerning the hypoglycaemic effect of PLR focused on herbal formulas containing PLR (eg, Gegen Qinlian decoction) or PLR extracts (eg, Puerarin injection). The indirectness of these trials in reflecting the effect and safety of PLR on T2DM should be negligible. In addition, previous studies also have substantial methodological limitations, such as the absence of blinding, failure to implement allocation concealment and irrational statistical analysis methods. In contrast, this RCT will adopt double-blinding for both patients and clinicians by establishing a placebo control, standardising the implementation of randomisation and allocation concealment procedures and undertaking rigorous quality control during the follow-up stage. In the statistical analysis, the main analysis will be based on the full analysis set constructed based on the modified intention-to-treat principle, which will obtain a relatively conservative effect estimate (ie, unfavourable to PLR). Moreover, we will fit appropriate multivariate models for different types of outcomes to overcome the potential confounding biases related to the centre effect and other covariates. These methodological advantages enable our study to yield accurate estimates regarding the effects of PLR.
Although herbal formulas containing PLR or puerarin injection may also have hypoglycaemic effects, it is necessary to investigate the individual hypoglycaemic effects of PLR. If this RCT ultimately demonstrates that the daily dose of PLR is effective and safe for T2DM patients, it will lead to multiple positive impacts. First, the supplementary hypoglycaemic effect of PLR will facilitate the control of blood glucose at an ideal level, delay the progression of T2DM and ultimately reduce the risk of diabetic complications. If PLR is verified to be safe, it could be used to replace some doses of hypoglycaemic drugs to mitigate adverse drug reactions. Owing to its medicinal and edible properties, PLR can be directly consumed as food or added to daily edible products such as tea or beverages in numerous countries, which will contribute to improving compliance with long-term use by patients with T2DM. In addition, PLR is an inexpensive herb. Consumed at the daily dose of this trial, the cost per month of PLR is only approximately US$2. Therefore, the complementary treatment of PLR may also assist in reducing the medical costs of patients with T2DM.
Although RCTs balance both known and unknown confounders across groups through randomisation, absolute residual imbalances may still compromise the accuracy of effect estimates. Therefore, adjusting for confounders remains necessary in RCTs, with their selection guided by clinical relevance rather than statistical criteria.40 41 Based on this principle, we prespecified age, body mass index, baseline HbA1c, use of insulin, research centre, time and time-by-group interaction as fixed-effect or random-effect covariates for adjustment. Given the substantial diversity of hypoglycaemic drugs and their potential combinations—which could generate dozens of categories—adjusting for all drug types as confounders would introduce the curse of dimensionality, data sparsity and overfitting, thereby undermining the reliability of effect estimation.42–44 To mitigate this, we dichotomised the hypoglycaemic drug factor into ‘insulin use versus non-use’, selecting this classification to maximise clinical heterogeneity. Should any specific hypoglycaemic drug categories demonstrate significant between-group differences in proportions, additional adjustments will be incorporated into sensitivity analyses.
One potential limitation of this study lies in the assessment of certain subjective outcomes, namely, the Diabetic Symptom Severity Grading Scale and the Diabetes-Specific Quality of Life Scale, which might be influenced by disparities in the comprehension of subjective items among participants or clinicians. To minimise deviation from cognitive factors, we will establish evaluation criteria for each scale item in the training of the standard operating procedure and guide the participants to accurately understand their condition. Another potential limitation is that some participants may find it difficult to adhere to the 12-week intervention and follow-up plan. To improve participant compliance, we will maintain communication with all participants throughout the follow-up process, answer patients’ consultations on the condition at any time and offer research subsidies for all participants and compensation of PLR samples for the placebo group. A third limitation pertains to the 12-week duration, which is insufficient to evaluate the long-term effects of PLR on T2DM outcomes; furthermore, HbA1c measurements at weeks 4 and 8 could be confounded by pretrial medication use. Should this trial demonstrate statistically significant efficacy in the primary outcome, we will conduct further real-world studies to assess the long-term efficacy and safety profile of PLR in T2DM management.
In summary, to date, no rigorous RCTs have assessed the effects of PLR on the clinical outcomes of patients with T2DM. This study will have a sufficient sample size to obtain accurate effect estimates and will conduct a follow-up for up to 3 months to verify the long-term safety and patient compliance with the daily consumption of PLR. The research findings may provide new options and evidence-based regimens for the management of T2DM.
Ethics and dissemination
The protocol has been approved by the Ethics Committees of the First Affiliated Hospital of Nanchang University (approval number: IIT(2024) LLS No.303) and the Affiliated Hospital of Jiangxi University of Chinese Medicine (approval number: JZFYLL2024006200087). The trial will be conducted in strict accordance with the Declaration of Helsinki and Good Clinical Practice standards. The patient informed consent form contains the process of the trial and the possible benefits and risks. Patients will receive free treatment if any intervention-related adverse effects occur. Details of the collection and use of participant data are described in the informed consent form (online supplemental file 1). The investigators will initiate the study with the patient after he or she has signed the informed consent form. Participants can voluntarily withdraw from the trial at any time for any reason without affecting subsequent treatment.
Supplemental material
We will disseminate the study findings through publications in peer-reviewed journals and conference presentations.
Trial status
The protocol version is 1.1, developed on 31 May 2024. The trial began recruiting on 25 July 2024 and is anticipated to finish in October 2025.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors JC and JW designed the study, developed the statistical analysis plan and drafted the manuscript. LY, QX, JZ, XY, QF, RF and CL were involved in the study design, communicated with the community centres and revised the manuscript; YZ and WZ provided critical methodological advice and revised the manuscript; ZL and XZ conceived and designed the study and reviewed the manuscript. XZ acts as the guarantor. All the authors contributed to the writing of the manuscript in detail and no professional writers were involved. All the authors have read and approved the final version of the manuscript.
Funding This work is supported by the National Natural Science Foundation of China (82274681), the Youth Talent Cultivation Project of the First Affiliated Hospital of Nanchang University (YFYPY202022), the Ganpo Talents Support Program: University Leadership Talent Cultivation Project (QN2023045), 2025 Central Fiscal Transfer Payment for Local Projects - Traditional Chinese Medicine Treatment of Dominant Diseases Project (Clinical Evidence-Based Capacity Improvement) and the Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (CXTD-22012). All funders had no role in the design of the study protocol and will have no role in the collection, analysis or interpretation of the data or in the writing of the manuscript.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.