Article Text
Abstract
Introduction This protocol assesses the effect of a wearable activity tracker-assisted physiotherapeutic intervention on postoperative outcomes in patients undergoing total knee replacement (TKR) surgery. Despite advancements in TKR technology, patient dissatisfaction remains a concern, with adherence to physical activity guidelines being particularly poor among those with knee osteoarthritis. The primary aim of this trial is to evaluate the effectiveness of a smart ring-assisted physiotherapeutic intervention in improving outcomes, as measured by the Oxford Knee Score (OKS), 12 months after a TKR.
Methods and analysis We are conducting this randomised controlled trial at Coxa Hospital for Joint Replacement in Finland, where participants are randomly assigned to either an intervention with smart ring-supported physiotherapeutic intervention or a usual care control group in a 1:1 ratio in patients undergoing TKR surgery. The primary outcome measure is the OKS at 12 months after surgery. Secondary outcomes encompass OKS variance, poor postoperative outcome (defined as ≤7 points change on the OKS), quality of life questionnaire EuroQol 5-Dimensions 5-Levels(EQ-5D-5L), knee range of motion, pain scores, patient satisfaction and healthcare resource utilisation. The study population comprises patients aged 18–70 undergoing unilateral primary TKR for knee osteoarthritis. Statistical analysis involves logistic regression and linear mixed models to assess group differences in outcomes over time, with adjustments for relevant covariates.
Ethics and dissemination The trial was approved by the Tampere University Hospital Ethical Committee (R22078) and participants are required to provide written informed consent. Procedures in conducting the trial are aligned with the principles of Good Scientific Practice outlined by the Finnish Advisory Board on Research Integrity. The results of this trial will be disseminated as a series of articles published in a peer-reviewed medical journal.
Trial registration number NCT05599776.
- Patients
- Knee
- Wearable Electronic Devices
- Randomized Controlled Trial
- Orthopedics
- ORTHOPAEDIC & TRAUMA SURGERY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Patients
- Knee
- Wearable Electronic Devices
- Randomized Controlled Trial
- Orthopedics
- ORTHOPAEDIC & TRAUMA SURGERY
Strengths and limitations of this study
The duration of the trial (12 months) is regarded as the gold standard for evaluating total knee arthroplasty rehabilitation.
The trial involves a multidisciplinary team, including orthopaedic surgeons, physiotherapists, nurses and a study nurse.
We aim to achieve a large sample size (338).
The trial exclusively focuses on smart rings and does not incorporate other types of wearable technology.
Recruiting for the trial can be challenging, as eligible participants, often closer to the maximum age (70), may struggle with using smart devices.
Introduction
Background
Knee osteoarthritis (OA) is a common and disabling degenerative condition. Total knee replacement (TKR) is an efficacious and cost-effective surgical procedure that decreases knee pain and improves physical function in patients with end-stage OA of the knee joint.1–4 Currently, the longevity of TKRs is not a clinical problem, as the majority of modern TKRs are expected to last more than 20 years.3 However, approximately 10–20% of patients report persistent pain and dissatisfaction with the postoperative outcome following their TKR.5–8 Despite the recent advances in TKR technology aiming to improve patient outcomes, such as robotic-assisted surgery, major breakthroughs have not been made regarding patient-reported outcomes of TKR.5–8
Physical activity (PA) is defined as ‘any bodily movement produced by skeletal muscles that results in energy expenditure’ by Caspersen et al.9 Regular PA has been proven to improve physical functioning, quality of sleep and quality of life, while it also prevents and reduces the risk of many diseases and conditions.10 According to the Physical Activity Guidelines for Americans, adults should engage in 150–300 min of moderate-intensity aerobic activity, or 75–150 min of vigorous-intensity aerobic activity per week, or a combination of both. In addition, they should perform resistance training at least 2 days/week.11 Recommendations also emphasise that moving more and sitting less, that is, avoiding sedentary behaviour, will benefit nearly everyone.11
Adherence to PA guidelines is poor among persons with knee OA.12 Additionally, according to a recent systematic review and meta-analysis based on objective accelerometer data, patients’ PA levels often remained unchanged at 6 months after the TKR surgery despite moderate to large reported improvements in pain, functioning and quality of life. Compared with preoperative PA levels, small to moderate increases in PA levels were found at 12 months after surgery.13
The use of activity trackers and wearable technology devices (wearables) offers potential ways to promote and increase PA in healthy adult populations.14 15 PA interventions using wearables have also been efficient in increasing PA in adults with musculoskeletal disorders such as arthritis and low back pain.16 17 Few studies of PA interventions used wearables, either alone or in combination with education or rehabilitation, in adults following TKR surgery.18 According to a systematic review by Master et al, based on three moderate-quality randomised controlled trials (RCTs), PA interventions that use wearables may have a positive impact on steps per day in patients after total knee or hip arthroplasty.18 Further research is needed since only a study done by Christiansen et al reported patient-rated or functional outcomes at 12-month follow-up, which is regarded as the gold standard in assessing the efficacy of TKR interventions.19 The most improvement in patient-reported physical function is likely to occur in the first 6 months after TKR, but further improvements in physical function may be seen between 6 and 12 months after surgery according to a prospective cohort study by Hamilton et al.7
Objectives
The primary objective of this RCT is to evaluate the effect of a smart ring-supported physiotherapy intervention, compared with the standard care received by the control group, on patient-reported outcomes. The primary focus is to assess differences in Oxford Knee Scores (OKS) between the groups 12 months after undergoing primary TKR. The key secondary objectives of this trial are to examine the outcome variance (OKS), poor postoperative outcome (defined as ≤7 points change on the OKS) and self-reported quality of life questionnaire EuroQol 5-Dimension 5-Levels (EQ-5D-5L) between the intervention and control groups at the same time point. Other secondary objectives include the assessment of active and passive knee range of motion (ROM), UCLA activity scale (UCLA), Forgotten Joint Score 12 (FJS-12), incidences of readmissions, rate of manipulation in anaesthesia and visits to the emergency rooms after the index TKR.
Methods and analysis
Study design
The study is a two-arm, 1:1 randomised trial with a target sample size of 338 participants, assigned to either a control group, receiving standard care after TKR, or an intervention group, receiving smart right-supported physiotherapy with the option to monitor in case of alarming metrics sent from the smart ring.
The study is a controlled, single-centre trial adhering to Standard Protocol Items: Recommendations for Interventional Trials guidelines. The study will be carried out at Coxa Hospital for Joint Replacement (Tampere, Finland), a university-affiliated, publicly funded orthopaedic hospital specialising in joint replacement surgery.
The trial has been registered in compliance with the WHO Trial Registry Data Set (online supplemental appendix A), ensuring alignment with international standards for clinical trial transparency.
Supplemental material
Protocol amendments
Since its inception in 2022, the protocol has undergone several significant modifications. Initially, patient and public involvement (PPI) was not included; however, in response to feedback from the peer review process, PPI was incorporated into the study as of December 2024.
Originally, the protocol focused on detecting poor postoperative outcomes as the primary objective. Based on recommendations from peer reviewers, this outcome has now been redesignated as secondary, and the new primary outcome is the OKS measured between the study groups.
Additionally, a Data Monitoring Committee (DMC) was not part of the initial design. Following further suggestions from the peer review process, a DMC was introduced in December 2024 to enhance trial oversight. More details regarding the DMC can be found in the ‘Data Management’ section of this protocol.
Although changing the primary outcome necessitated adjustments in the power calculations, the final estimates remain consistent with the original design, and the sample size has not been altered.
Furthermore, all these changes were discussed with the Chairman of the Tampere University Hospital Ethical Committee, who confirmed that the modifications do not affect the ethical conduct of the trial, and as such, no additional formal review or processing was required.
Randomisation
The randomisation is conducted electronically through the Research Electronic Data Capture (REDCap) software, with ongoing recruitment and baseline assessment of new participants. Participants are allocated in a 1:1 ratio using variable block sizes, without any stratifications for group assignments.
Baseline assessment
The baseline assessment evaluates various factors to ensure a thorough understanding of the participant’s condition and background. Key elements include sex, age, height and weight, providing essential demographic context. Additionally, details such as which knee is affected (left or right knee) and any prior interventions or treatments, including physiotherapy, medical therapy, cortisone or hyaluronate injections and endoscopy, are noted.
Socioeconomic factors such as occupation and education levels are clarified, and smoking history and work-related evaluations are also asked. Participants are also asked to evaluate their occupation’s physical demands and ability to perform at work. Assessment considers various work situations, such as whether the participant is on sick leave, partial sick leave or fully operational.
Furthermore, the participant’s quality of life and overall well-being are assessed using standardised measures, such as the EQ-5D-5L scale.20–22 Additionally, the participant’s perception of pain and functional limitations is gauged through the visual analogue scale (VAS), both during leisure activities and exertion.
Outcomes
Primary outcome
The primary outcome is the OKS measured on the Finnish language version of the OKS at 12 months after surgery.23
Key secondary outcomes
The key secondary outcome measures are the proportion of patients with poor postoperative outcome (7 points or less change) on the OKS 12 months after surgery, homogeneity of variance in the OKS and difference in quality of life measured with EQ-5D-5L 12 months after the surgery.23 24
Secondary outcomes
Secondary outcomes include primary and key secondary outcomes measured at 3 and 6 months after the index surgery. Other secondary measured outcomes both preoperatively and after surgery at 3, 6 and 12 months are the FJS-12, EQ-5D-5L and pain at rest and walking using the pain VAS. Active and passive knee ROM is measured at 3 and 12 months. Patient satisfaction VAS and accepted symptom state questions will be measured 12 months after surgery. The rate of manipulation under anaesthesia, readmissions within 90 days and the visits to the emergency department during the 12-month follow-up after surgery are also recorded.
Study population
The study population consists of patients undergoing unilateral primary TKR surgery for primary knee OA in the study hospital.
Recruitment and consent
Recruitment for the study commenced in November 2022 and is expected to conclude by December 2026, with the study anticipated to be completed by December 2027. Patients will be informed of the trial and invited to participate preoperatively at the outpatient clinic when they undergo clinical assessment of eligibility to undergo TKR surgery. This assessment will be carried out by experienced orthopaedic surgeons in the study group. At study entry, a suitably qualified member of the research team will obtain written informed consent.
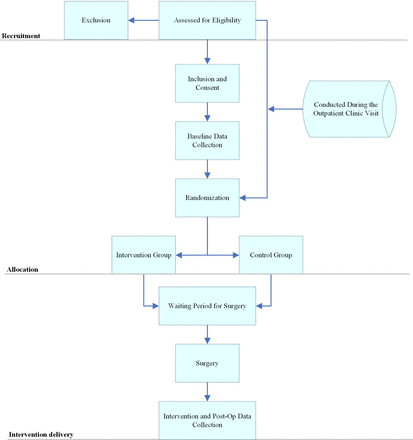
The participant consent form (online supplemental appendix B) informs participants that their participation is voluntary, and they have the right to refuse treatment or withdraw consent at any point during the study without providing a reason. Recruitment, baseline data collection, randomisation and their timing relative to surgery are outlined in figure 1.
Supplemental material
Supplemental material
Flow chart illustrating the timing of recruitment, allocation and intervention delivery. This flow chart outlines the study process from recruitment through intervention delivery. Participants are assessed for eligibility. Those eligible provide consent and undergo baseline data collection during an outpatient clinic visit. Following randomisation, participants are allocated to either the intervention or control group. Both groups experience a waiting period before surgery, after which intervention and postoperative data collection occur. The structured approach ensures clear delineation of participant flow and study methodology.
Potential recruitment bias
We acknowledge that requiring participants to use a wearable device and app may introduce recruitment bias, favouring younger or more technologically literate individuals and potentially under-representing older adults. While this is not purely a pilot study, the trial aims to assess the feasibility and potential of wearable technology in postoperative care, with findings primarily informing future research rather than broad generalisability. However, the results of the study will be generalised to the patient group of the same age.
To mitigate recruitment bias, we will offer guidance to all participants during the preoperative assessment to ensure they are comfortable with the wearable device and app. We will also actively promote diversity in the study population by targeting recruitment efforts across a broad age range and monitoring the age distribution throughout the recruitment process to identify and address any emerging trends in bias. Finally, we will analyse potential recruitment bias by comparing the demographics of the study population, particularly age distribution, to the general TKR population and documenting any observed differences in representativeness.
Adherence and discontinuation
We expect high adherence to the trial because the postoperative care provided, such as X-rays and physiotherapeutic interventions, is standardised and proven effective for TKR, irrespective of the trial. This care is also affordable, which further supports adherence. Additionally, participants will use a smart ring that automatically stores data if connected to their phone and the Oura app is opened. Their responsibility will be to keep the ring charged. One factor that may affect adherence is the requirement for participants to manually complete the questionnaires for both primary and secondary outcomes.
Adherence to trial processes is evaluated based on participants completing scheduled questionnaires and attending follow-up visits. Adherence to trial interventions focuses on compliance with the physiotherapy programme and consistent use of the smart ring. By distinguishing these two aspects, the study aims to better understand participant engagement and its influence on trial outcomes.
Participants can withdraw from the trial at any time without providing a reason. If consent is revoked or participation discontinues for any reason, the data collected up to that point may still be used for research, provided it is necessary for completing the trial and is permitted by legislation.
Study eligibility
Inclusion criteria
Patients undergoing primary total knee arthroplasty (TKA) for primary OA.
Patients able to consent and willing to comply with the study protocol.
Patients aged 18–70 years.
Patients are able to use a smartphone and a smart ring.
Exclusion criteria
Patients unwilling to provide informed consent.
With >15 degrees varus or valgus, or >15 degrees fixed flexion deformity.
Physical, emotional or neurological conditions that would compromise the patient; for example, poor compliance with postoperative rehabilitation and follow-up (eg, drug or alcohol abuse, serious mental illness, general neurological conditions, such as Parkinson, Multiple sclerosis, etc).
Patients unable to attend the study physiotherapy appointments at the outpatient clinic.
Patients unable to wear the Oura ring (eg, in case of OA or rheumatoid arthritis in the finger joints).
Patients with cardiac arrhythmia.
Interventions
The primary goals of the postoperative physiotherapy care after TKR are:
To improve active knee ROM: the objective is to achieve full knee extension and at least the preoperative knee flexion.
To enhance muscle strength: specifically, the focus will be on the quadratus femoris muscle, starting with isometric exercises and progressing to more functional exercises.
To achieve fluent and normal gait: this will be accomplished through gait and balance exercises to help the patient regain a normal walking pattern.
Intervention group
Participants in the intervention group will receive standard postoperative physiotherapy care, which includes both face-to-face physiotherapy and the use of a smart ring (Oura) for monitoring PA. The initial baseline assessment will be conducted by either a study nurse or a physiotherapist, provided the participant consents to take part in the trial. This consent is typically obtained after the first physician visit, which occurs during the planning phase of the TKR procedure (a process that may span from weeks to months before surgery).
Following surgery, participants will meet with physiotherapists in the ward, who are not exclusively designated to the trial, to receive guidance on how to perform exercises prior to discharge. They will continue the exercises at home. The intervention group will attend four physiotherapy visits during the postoperative phase:
Immediate postoperative visit: this will take place in the ward immediately after surgery.
Four-week visit: this visit will be conducted by a physiotherapist at the participant’s local health centre, occupational health clinic or private physiotherapy clinic. Participants are instructed to schedule these appointments independently.
3-month and 12-month visits: conducted by one of three designated study physiotherapists at the Coxa outpatient clinic.
In addition to physiotherapy, the intervention group will use a smart ring (Oura) to monitor physical fitness, activity levels, readiness scores and sleep quality. The smart ring will be provided during the preoperative phase to establish baseline data. It will be worn throughout the preoperative and postoperative phases:
Preoperative use: the smart ring will gather baseline data on activity and sleep.
Postoperative use: the smart ring will be returned during the 3-month postoperative visit, and participants will receive it again 4–6 weeks before the 12-month follow-up to evaluate changes in physical fitness and sleep patterns.
Control group
Participants in the control group will undergo the same standard postoperative physiotherapy care as the intervention group, including four physiotherapy visits. However, they will not use the smart ring for postoperative monitoring.
Before surgery, control group participants will be provided with a smart ring to collect baseline data on physical fitness and activity levels. The smart ring will be returned during their hospital stay for the surgery. Both the control and intervention groups will follow an identical physiotherapy visit schedule.
Data collection processes
Data collection will occur at several time points throughout the trial. Both patient-reported outcomes and physiological measurements will be used to assess the outcomes.
Preoperative and postoperative data collection
Participants will complete baseline assessments before surgery. After surgery, they will complete questionnaires at 3, 6 and 12 months. Active and passive ROM will be assessed during the baseline assessment, as well as at the 3 and 12-month visits, as detailed in online supplemental appendix C.
Supplemental material
Smart ring data collection
The smart ring (Oura) will collect data on the participant’s PA, sleep quality and readiness scores. These data will be used to assess changes in physical fitness and recovery progress over time. The intervention group will wear the smart ring during both the preoperative and postoperative periods, and the data will be analysed accordingly.
Monitoring and follow-up data
Weekly monitoring: monitoring of participants will begin immediately after surgery through the Oura Teams platform. Physiotherapists will use this platform to track the participant’s PA and sleep patterns. Patients have access to view their own activity parameters. The data collected, which will be used for exploratory secondary analyses, are described in online supplemental appendix C.
Contact criteria
Participants will be contacted if they have not attained 50% of their preoperative activity level, as indicated by step counts and activity scores, within 4 weeks after operation.
Participants will also be contacted if their activity level falls below 50% of their previous week’s activity.
Exclusion criteria
Participants will not be contacted solely based on their activity, passivity and sleep data if it remains stable or improves over time, without any concerning trends observed.
Participant timeline
Prior to surgery, patients will complete the OKS questionnaire. If eligible, they will also complete the EQ-5D-5L, FJS-12, Connor-Davidson resilience and life optimism questionnaires. These assessments are explained in more detail in online supplemental appendix D.
Supplemental material
After surgery, participants in the intervention group will receive the smart ring (Oura) 4–6 weeks before their 12-month follow-up visit and will wear it until the 3-month visit. The monitoring and data collection associated with the smart ring are described in the Data collections processes section. Monitoring will conclude following the initial 3 months after surgery.
The 12-month follow-up period begins on the date of surgery to ensure standardised outcome measurement. The mean waiting time for surgery is approximately 3 months after recruitment. If a patient does not undergo surgery, they will be excluded from the study, and any data collected prior to surgery will not be included in the analysis. A detailed overview of the participant timeline, including the schedule for assessments and interventions, is provided in online supplemental appendix C.
Study assessments
Study assessments are written in detail in online supplemental appendix D.
Sample size calculation
The primary outcome of this study is the OKS assessed 12 months after surgery. We aim to detect a minimum difference of 2.5 points, which represents the smallest meaningful change we do not want to miss and is half of the standard minimally clinically important difference for the OKS. This threshold is based on the high mean OKS observed at Coxa Hospital.25
At Coxa Hospital, the average 12-month OKS is 41.4, with an SD of 6.7. As of 10 March 2025, our database includes 11 234 patients who received one of the two most commonly used cruciate-retaining prostheses, with complete preoperative and 12-month OKS assessments.
To achieve sufficient statistical power (90%) with a type 1 error probability of 5% and accounting for a 10% attrition rate, our calculations indicate that 169 patients per group are required to reliably detect the targeted difference.
Statistical analysis
The primary outcome of our study is the OKS score at 12 months after surgery. This is estimated as a marginal difference in the proportions between study groups from a logistic model. Age, gender and baseline OKS are used as covariates in this model. Each time point is analysed separately. All other binary outcome variables are analysed in a similar fashion. Continuous outcomes are analysed with a linear mixed model as the full analysis set. No missing data are imputed. For each outcome, age, gender and respectively baseline values are used as covariates. The marginal effect of group baseline values is the difference at each time point, estimated with 95% CIs. Variance in the OKS between study groups is analysed using the F-test.
The analysis will follow an intention-to-treat approach, ensuring that all randomised participants who undergo surgery are included. Sensitivity analyses will be conducted to assess the impact of any deviations, such as delays between randomisation and surgery, on the study results. Participants who do not undergo surgery will be excluded from the study, and any data collected prior to surgery will not be included in the analysis. Analyses will be done using RStudio.
Futility assessment
When half of the patients have been recruited (ie, 169 patients), the DMC will be consulted to assess the feasibility and futility regarding the chances of meeting a desired proportion of patients with contact during postoperative surveillance. In collaboration with the DMC, we will evaluate the study data, including patient contact patterns recorded by the smart ring monitor, and conduct an interim futility assessment.
Data management
Each patient will be assigned a unique trial identification number (TIN) that will be matched with the patient’s ID. The matching key will be stored in a locked office of the study nurse in the Coxa Hospital. After the recruitment of the patient has been carried out, the identification of each patient will only be possible after retrieving the matching key. Throughout the trial, the research data will be handled only with a TIN. The research data will be saved to a database with an online patient management program REDCap (https://www.project-redcap.org/), which is secured by password and personal identification sign-on method. Only researchers will have access to REDCap data. It is a secure research server provided by Tampere University Hospital. A DMC has been established for the trial and will be consulted at the midpoint of recruitment, in accordance with the futility assessment. The DMC will consist of three independent medical doctors, unaffiliated with the trial sponsor and without competing interests. Further details, including the names and affiliations of the committee members, are provided in online supplemental appendix E.
Supplemental material
All primary and secondary data will be acquired and stored on the study trial server. Data entry will occur either by the patient or a study nurse during the initial visit using tablets, or by computer after completing the baseline assessment on paper. Patients will receive a link via email during the follow-ups. Patient-reported outcome data will be entered directly in REDCap by the patients with the ‘required fields’ option activated to ensure no missing items from completed questionnaires. The server has been approved by an information security committee at Tampere University Hospital. At the end of the trial, each researcher will have access to the data for further analyses. The individual patient data acquired at different time points will be saved in Comma Separated Values format, which is transferrable to, for example, Microsoft Excel. All variables in the dataset will be described and suitable metadata standards will be used when available.
Smart ring data are stored in a separate server owned by Oura company, the smart ring manufacturer. Data are entered to a Teams online platform in which the patient is instructed to register as a user using a preferred email address. TIN is used as identification without additional personal detail. After the study is completed, these data are transferred and matched to REDCap data using the TIN.
The copyright of the trial research data will be owned and created by the collaboration parties. All participating researchers will receive access to the data after the trial is completed. Due to confidentiality and legal agreements, public data sharing will be restricted until primary analysis and publication have been done. Under certain circumstances, for example, when a new member joins the collaboration, we will grant access to the data. All data will be saved for 5 years after the end of the trial.
Blinded interpretation of the results
The intention-to-treat analysis will be interpreted while remaining blinded. This blinded analysis will focus on the primary outcome and key secondary outcomes. A junior researcher will export the data from REDCap and provide the blinded dataset to the statistical analyst. The analysis results comparing treatment A and treatment B will be presented to the project group, leading to the development of the blinded interpretation. In this process, Interpretation Version 1 will assume that group A received the active intervention, while Interpretation Version 2 will assume that group A was the control group. A draft of the blinded interpretation will be circulated for approval among all coauthors. No other outcomes will be analysed until all coauthors agree on the blinded interpretation. Any additional analyses specified in this document will be conducted after the blinded interpretation is published.26
Safety considerations
This study involves routine TKR surgery in patients aged 18–70 years for primary knee OA. There are no additional risks to patients when participating in the study. Patients will undergo a TKR as per standard management regime. Patients will be informed of the standard risks associated with anaesthetic and knee replacement operations.
Possible complications and consequences related to the index surgery include a risk of anaesthesia-related problems, death, morbidity including wound infection, bleeding intraoperatively and postoperatively, thromboembolic complications and complications secondary to existing comorbidity, for example, ischaemic heart disease. Specific complications following knee replacement procedures include loosening of components, dislocation of the knee or polyethylene liner, superficial and deep infection, unexplained knee pain, knee stiffness, postoperative haematoma, mechanical failure of replacement and periprosthetic fracture. These complications may lead to further surgery such as revision operations, washout, manipulation under anaesthetic, aspiration or patella resurfacing.
A serious adverse event (SAE) is defined as any adverse event (AE) during the course of the study resulting from the administration of any of the research procedures required by the protocol. An event qualifies as an SAE if it results in death, is considered life threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, or results in persistent or significant disability. Additionally, events that may not result in death, are not life threatening or do not require hospitalisation may be considered as SAEs if, based on appropriate medical judgment, they may jeopardise the participant and may require medical or surgical intervention to prevent one of the outcomes listed above. AEs occurring during the trial will be recorded at in-person visits up to the 12-month follow-up. These events may include complications related to surgery, physiotherapy and other trial procedures. A comprehensive list of AEs will be provided and analysed in the final paper, which will present the trial’s results. Comorbidities such as cancer, depression, diabetes and stroke, for example, may influence short-term risks like hospital readmissions. However, in the long term, while these conditions may impact safety through an increased incidence of AEs, they are unlikely to significantly affect the effectiveness of TKA.27
Patient and public involvement
Patient involvement was incorporated into the trial in December 2024 as part of an evolution in the study design. This addition aims to enrich the study’s overall outlook by integrating patient perspectives, ensuring that the strengths and weaknesses of the trial are more comprehensively understood. This inclusion aims to leverage the lived experiences of patients to benefit future individuals facing similar circumstances. A comprehensive explanation of the patient involvement process is provided in online supplemental appendix E for further reference.
Ethics and dissemination
Ethical approval for this study was obtained from the Tampere University Hospital Ethical Committee (Institutional Review Board No R22078). The trial is funded by Coxa Hospital for Joint Replacement. The trial is registered with ClinicalTrials.gov (NCT05599776). In the implementation of the research, all procedures adhere to the principles of Good Scientific Practice as outlined by the Finnish Advisory Board on Research Integrity. Participants are required to provide written informed consent.
Data collected from participants is necessary for conducting the trial, and participant identities are known only to the researchers involved, who are obligated to maintain confidentiality. All personally identifiable information, such as social security numbers and names, will be removed and replaced with code numbers. The data will be stored separately from the coded information and will not be disclosed to individuals outside the research team. The study results will primarily be reported at the group level, ensuring that individual participants cannot be identified from the publications or reports of the research findings.
There is no immediate benefit in participation in this trial. However, participants will have access to a smart ring (Oura) monitoring their recovery, providing them with more information about their recovery process based on objective measures.
The risks associated with the trial are the same as those associated with a standard joint replacement surgery procedure. In addition, the use of the Oura ring may pose a risk of patients striving for excessive activity immediately after surgery, potentially leading to increased pain and swelling in the operated limb. This risk is mitigated by guiding patients to move within their own comfort levels and limits of recovery, regardless of any activity targets suggested by the Oura ring.
In the event of an unexpected negative outcome, the principal researcher will promptly contact the participant to discuss the continuation of the study. If any harm is caused by a procedure due to the trial, participants may apply for compensation, as they are covered by public health insurance. For reasons other than harm caused by the trial, participants will be directed to seek compensation from the research centre’s health insurance. Additionally, any important protocol amendments will be communicated by the principal researcher to investigators, trial participants, registries and journals.
The writing committee responsible for reporting the data will be appointed by the principal researcher, and no professional writers will be used. The findings from this trial will be disseminated through a series of articles published in a peer-reviewed medical journal.
Ethics statements
Patient consent for publication
Acknowledgments
The authors express their sincere gratitude to Sakke Purolainen, statistician at the University of Tampere, for his commitment to serving as the trial statistician when needed, particularly during the final data analysis phase.
References
Footnotes
Contributors JH: writing and submission of the protocol. LK: planning and writing the protocol. AR: planning, writing and reviewing the protocol. VM, AE: reviewing and commenting on the protocol. AR is the guarantor of the trial. ChatGPT was occasionally used to refine sentences and small sections of chapters, enhancing the overall fluency of the text.
Funding The trial was funded by the Coxa Hospital for Joint Replacement. The study is partly funded by the Finnish Government Research Funding.
Disclaimer None of the funders will be involved in the collection, management, analysis, or interpretation of data, nor in the writing of the report or the decision to submit the report for publication.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.