Article Text
Abstract
Objective The aim of this study was to compare in-hospital mortality across waves in patients without and with a ceiling of care at hospital admission.
Design A multicentre prospective cohort study.
Setting Five tertiary hospitals in Catalonia, Spain, during four waves of the COVID-19 pandemic. Data from the first wave embraced from March to April 2020, second wave from October to November 2020, third wave from January to February 2021 and fourth wave from July to August 2021.
Participants All consecutive adult subjects (older than 18 years old) admitted to any of the five aforementioned centres. All subjects had a confirmed SARS-CoV-2 infection (with a positive PCR test or antigen test) and an overnight hospital stay. Ceiling of care defined as the highest level of care that a patient will receive during medical treatment was assessed at hospital admission for all patients.
Primary measure In-hospital mortality.
Results A total of 3982 hospitalised patients without ceiling of care and 1831 hospitalised patients with ceiling of care were included in the analysis. The adjusted ORs of in-hospital mortality in the second wave were 0.57 (95% CI 0.40 to 0.80), in the third 0.56 (95% CI 0.37 to 0.84) and in the fourth 0.34 (95% CI 0.21 to 0.56) compared with the first wave in subjects without ceiling of care. The adjusted OR was significantly lower in the fourth (0.38, 95% CI 0.25 to 0.58) wave compared with the first wave in subjects with ceiling of care.
Conclusions In patients without ceiling of care, mortality decreased over time, suggesting better disease knowledge and management. In ceiling of care, only fourth wave patients were less likely to die than first wave patients. In a future infectious disease pandemic, it will be a challenge to improve the management of patients with ceiling of care.
- COVID-19
- INFECTIOUS DISEASES
- PALLIATIVE CARE
- Epidemiology
Data availability statement
Data are available upon reasonable request. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is a multicentric study with a large number of subjects included from four different waves of the COVID-19 pandemic.
Several methods were used to compare in-hospital mortality between waves to increase the robustness of the estimated effects.
Despite the inverse probability weighting analysis, there may be unobserved characteristics that lead to residual confounding.
The national vaccination campaign started for the elderly subjects before the fourth wave, so it could not be used in the adjustment analysis.
Introduction
Despite the lack of definition in epidemiology, the term epidemic wave implies a natural pattern of peaks and troughs in the incidence of cases or hospitalisations due to an outbreak.1 Epidemics often occur in local or global waves, each one with variations in severity or in transmission dynamics.2–4
Following a similar pattern, the COVID-19 pandemic began in Wuhan, China, in December 2019, and spread rapidly across Europe, with the first outbreak in Italy in February 2020. During the course of the pandemic, countries and regions experienced several waves with distinct peaks in cases. In Spain, seven waves of the pandemic have been recorded between March 2020 and September 2023, with almost 14 million confirmed cases and more than 120 000 deaths.5 Throughout this period, knowledge of the disease has progressively increased with the sequencing of the virus,6 clinical trials to assess treatments efficacy,7 8 the identification of different strains of the virus9 and the development of vaccines.10 All these factors, together with the natural immunity protection against COVID-19,11 lead to a reduction in the need for hospitalisation, in-hospital mortality and complications.
The therapeutic ceiling of care refers to the highest level of care that a patient will receive during medical treatment. In general, in a non-pandemic setting, decisions about the ceiling of care are common practice when dealing with patients with a critical prognosis and have implications for the use of life-sustaining measures such as intubation, mechanical ventilation and cardiopulmonary resuscitation. However, in the peaks of the COVID-19 pandemic, decisions about the maximum level of care that each patient should receive, besides the critical prognosis of the patient, were made in a scenario of emergency with excess demand for critical care and limited availability of clinical resources. Previously published data12 13 suggest that COVID-19 hospitalised patients who had a ceiling of care were mainly older and had more comorbidities and higher incidence of in-hospital death. In-hospital mortality has been shown to decrease over time.14 15 However, little is known about the impact of ceiling of care on mortality in hospitalised patients with COVID-19 across pandemic waves. Stratifying by care limitations helps to distinguish whether the reduction in mortality was due to advances in intensive care unit management, improved general hospital care or shifts in decision-making. This approach addresses a gap in previous research, which has often overlooked how changes in patient selection for intensive care can bias mortality trends. Understanding these dynamics can inform clinical decision-making and ensure optimal management for all patients, regardless of their care limitations.
Our hypothesis is that the decrease in in-hospital mortality over time is different in patients with and without ceiling of care. The aim of this study was to compare in-hospital mortality across four COVID-19 waves between patients with and without a ceiling of care at hospital admission.
Methods
Study design and setting
The MetroSud study is an observational multicentre study conducted in five centres located in the southern metropolitan area of Barcelona (Catalonia, Spain), to characterise all patients with COVID-19 admitted to these hospitals during four waves of the pandemic from March 2020 to August 2021. COVID-19 epidemic waves followed a pattern of peaks and troughs in the incidence of cases or hospitalisations. There is no official date for the start or end of a wave in Catalonia, but recruitment in the MetroSud cohort occurred during peaks in the incidence of cases or hospitalisations in our hospitals. The Infectious Diseases Unit of Bellvitge’s Hospital developed the protocol for this study as soon as the first cases appeared. After the first wave, and with the experience gained, the protocol and data collection were reactivated when early epidemiological indicators signalled the arrival of the second wave. This approach was continued in subsequent waves. Analysed data of the first wave of the COVID-19 pandemic embraced from March to April 2020, second wave from October to November 2020, third wave from January to February 2021 and fourth wave from July to August 2021.16 MetroSud cohort has been previously described.12
Eligibility criteria
The MetroSud cohort included all consecutive adult subjects (older than 18 years old) admitted to any of the five aforementioned centres. All subjects had a proven SARS-CoV-2 infection (with a positive PCR test or antigen test).
Data sources and study variables
An electronic case report form (eCRF) in REDCap17 was designed in March 2020 to collect study data: in-hospital mortality as main outcome, ceiling of care and epidemic wave as main independent variables, and subjects’ clinical profile to adjust for potential confounding.
Demographic data (age, sex, race), comorbidities and other relevant findings on medical history, previous medications, clinical symptoms, vital signs (body temperature, FiO2, O2 saturation, blood pressure, pulse and respiratory rate), laboratory results (D dimer, C-reactive protein, lactate dehydrogenase, leukocytes, and others) and respiratory exploration (wheezing, rhoncus), Pneumonia Severity Index (PSI) and ceiling of care were collected at baseline by the attending physicians. Patient status at hospital discharge was also recorded in the eCRF. No variables were transformed, and ranges of plausible values for continuous variables were indicated in the eCRF to ensure data quality.
The presence or absence of ceiling of care was decided at the emergency room by the attending physicians according to their criteria, taking into account the patient’s potential benefit of intensive treatments. In the beginning of the first wave, due to the intensive care unit (ICU) demand and capacity, the availability of resources at each participating hospital was also taken into account. Patients without a ceiling of care would have access to an ICU or could receive invasive mechanical ventilation. Otherwise, patients assigned to ceiling of care would have limited access to the ICU and, if they required any respiratory support, it would be non-rebreather mask, high-flow nasal cannula or non-invasive mechanical ventilation (NIMV). Information about ceiling of care was registered in the eCRF at hospital admission. Patients without information on ceiling of care assigned were excluded from the analysis.
Outcome variable
The outcome variable was in-hospital mortality defined as death by any cause during hospitalisation and was registered in the eCRF. Patients without information on in-hospital mortality were excluded from the analysis.
Statistical methods
To describe cohort characteristics, categorical variables were presented as the number of cases and percentage, while continuous variables were expressed as the mean and SD or median and IQR. All analyses were presented by wave and stratified by ceiling of care.
A pool of essential variables to describe the baseline profile of patients was defined. This pool included age, sex, Charlson score, ceiling of care, and circumstances at discharge. Patients who had incomplete data on this pool of variables were discarded from the analysis.
Once the variables to be used to match patients were identified, multiple imputation with chained equations (MICE)18 was used to create five datasets with complete data. Missing data were assumed to be at random. Predictive mean matching was used to impute continuous variables and binomial logistic regression was used to impute binary variables. Information on age, sex and baseline comorbidities (completed for all patients after exclusions) was used to impute missing values for obesity, body mass index (BMI), race, PSI, FiO2, oxygen support, D-dimer, C-reactive protein, leukocytes, haemoglobin and lymphocytes. Final estimates were adjusted for variability between the five imputed datasets according to the Rubin rules19 to obtain the final model.
With the database with all the missing data imputed, three models were constructed to study the association between in-hospital mortality and wave: (1) a crude logistic regression model using wave as a covariate, (2) a fully adjusted logistic regression model and (3) an inverse probability weighting (IPW) logistic regression model.
After discussion with clinicians, the variables included in the fully adjusted logistic regression model to minimise confounding and make patients comparable between waves were baseline variables that define the patient’s status at hospital admission: age, sex, race, BMI, obesity, long-term facility, comorbidities (diabetes mellitus, chronic obstructive pulmonary disease (COPD), heart failure, hypertension, renal insufficiency, dyslipidaemia, coronary heart disease, haematological neoplasm, solid neoplasm, organ transplantation, immunosuppressive treatment, chronic complex patient and patients with advanced chronic disease), baseline laboratory values (dimer, C-reactive protein, leukocytes, haemoglobin, lymphocytes), PSI, FiO2 and oxygen support.
IPW20 was used to adjust for differences in the patient baseline profile between waves. Bayesian additive regression trees, entropy balancing, generalised boosted models and generalised linear models were tested as methods for weighting individuals. In the end, we chose the method with better covariate balance between waves after weighting, which was the Bayesian additive regression trees method.21 In each imputed dataset, weights were calculated with the wave as the outcome and the variables used for the full adjusted logistic model as covariates.
To identify imbalances between waves after weighting, we estimated and described the standardised mean differences in baseline variables before and after weighting. We then fitted a logistic regression model for each imputation with in-hospital death as the outcome, using the stabilised weights and model-robust standard errors and adjusting for the variables that remained imbalanced between groups after weighting.
To overcome the limitation of assuming missing at random, a sensitivity analysis was performed by repeating the analyses using only those patients who had complete information on all variables.
We used the Strengthening the Reporting of Observational Studies in Epidemiology cohort checklist22 when writing our report. All analyses were performed with a two-sided significance level of 0.05 using R software V.4.3.0.23 The main R packages used for data management and analysis were flowchart,24 REDCapDM,25 mice,18 WeightIt,26 cobalt27 and survey.28
Patient and public involvement
There was no patient or public involvement in the development of the research design or in conducting the study.
Results
Flow chart
A total of 4417 patients without ceiling of care and 2159 patients with ceiling of care were included in the MetroSud. Patients who were discharged or died within 24 hours of admission (n=494 and n=15, respectively) were not considered hospitalised for the purposes of this study, in accordance with the study protocol. For those discharged within 24 hours, it was assumed that their clinical condition may have been more appropriately managed in an outpatient setting. In both cases, however, key study variables were often unavailable or incomplete, limiting the ability to include them in the analysis. Patients with incomplete data on a pool of essential variables (age, sex, Charlson score, ceiling of care and circumstances at discharge) (n=204) or patients who were initially admitted to one hospital but transferred to another and treated in the latter (n=48) were also excluded. After exclusions, a total of 3982 patients without ceiling of care and a total of 1831 patients with ceiling of care were included in the analysis. All patients were followed up until in-hospital death or hospital discharge (figure 1, flow chart).
Flow chart of the included patients without ceiling of care (left) and with ceiling of care (right).
Baseline characteristics by wave
Table 1 describes the baseline characteristics of the included patients by wave and stratified by ceiling of care. Other variables included in the matching process are described in online supplemental table 1.
Supplemental material
Patient’s most relevant characteristics according to wave and ceiling of care
Regarding age, patients with a ceiling of care were, in median, 20 years older than patients without a ceiling of care in all waves. There were no differences in the proportion of women. The most common race was Caucasian (in all waves, almost 90% of patients without ceiling of care and over 70% of patients with ceiling of care were Caucasian). Patients with a ceiling of care had a median Charlson Index more than 3 points higher than patients without a ceiling of care in all waves. PSI scores for patients with ceiling of care were more than 35 points higher in all waves (greater differences in wave 4) than PSI scores for patients without ceiling of care.
In-hospital mortality
The overall cumulative incidence of in-hospital mortality for patients with and without ceiling of care in all waves is shown in table 2.
Cumulative incidence and 95% CI for in-hospital mortality according to wave and ceiling of care
About 1 in 10 patients without ceiling of care died in hospital in the first and second waves. In patients with a ceiling of care, about 4 in 10 patients die in hospital in the first three waves. The percentages are lower in the fourth wave (5% and 30%, respectively, for patients without and with a ceiling of care).
Mortality in patients without ceiling of care
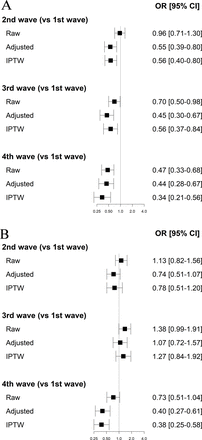
Figure 2A shows the balance of covariates before and after IPW by means of the standardised mean differences (SMD) in patients without a ceiling of care. The SMD for PSI remains above 0.2. To correct for this imbalance, PSI was included in the weighted mortality models. The ORs of the three models for mortality are shown in figure 3A. The results with the three methods are consistent and show the same trend for all waves. Patients from waves 2, 3 and 4 were less likely to die in hospital than patients from wave 1 both in the raw models and in the models adjusted for covariates or adjusted with weights (OR for all models and all waves lower than 1). In addition, the value of the OR decreases across waves.
Maximum standardised mean differences (SMD) before (Unweighted) and after weighting (Weighted) across waves for patients without a ceiling of care (A) and patients with ceiling of care (B). The SMD compares the difference in means between all pairs of waves in SD units. BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLP, dyslipidemia; DM, diabetes mellitus; MACA, patients with advanced chronic disease; PCC, chronic complex patient; PSI, Pneumonia Severity Index.
OR for raw, adjusted and inverse probability treatment weighting (IPTW) models for in-hospital mortality in patients without a ceiling of care (A) and with ceiling of care (B).
Mortality in patients with ceiling of care
Figure 2B shows the balance of covariates before and after IPW by means of the SMD in patients with a ceiling of care assigned at admission. Age, PSI and race showed a difference between waves greater than 0.2. These variables were included as adjustments in the weighted mortality model to account for these differences.
The ORs of the three models for mortality are shown in figure 3B. No differences were found between first and second wave patients or between first and third wave patient (neither in the crude nor in the adjusted models). For wave 4, both adjusted and IPW models showed that, given two patients with the same baseline profile, a wave 4 patient was less likely to die in hospital than a wave 1 patient.
Sensitivity analysis
To account for the assumption of missing at random, we perform a sensitivity analysis using only those patients who had all the variables completed. The results were in the same direction as for the imputed database. In patients with ceiling of care, the effect of the wave in mortality was the same in patients with and without complete data. In patients without ceiling of care, the effect was also similar, but as the sample size of the cohort with complete data was smaller, the OR for the second and third waves did not reach statistical significance.
Discussion
Our multicentre cohort study compared in-hospital mortality across COVID-19 waves between patients with and without a ceiling of care at hospital admission. We found that among patients without ceiling of care, those admitted in the first wave had worse in-hospital mortality than patients hospitalised during the other waves. Moreover, the adjusted models showed a significant decrease in mortality as the waves progressed. Among patients with a ceiling of care, no differences in in-hospital mortality were found between second and first wave patients, or between third and first wave patients. Only in the fourth wave, patients were less likely to die than first wave patients after adjustment for baseline risk. The magnitude of this effect on mortality reduction observed in patients with ceiling of care in the fourth wave was similar to the effect observed among patients without ceiling of care in the same fourth wave.
It is worth noting that if the differences in mortality between waves were only due to patient’s risk profile, the mortality rates would be similar after adjustment for baseline profile. However, this is not the case, as figure 3A shows that in the adjusted and weighted models, mortality among patients without ceiling of care decreases as waves progress in time (OR decreasing from 0.56 (second and third waves) to 0.34 (fourth wave) when comparing with the first wave). The emergency situation experienced by the hospitals in the first months of the pandemic, with a lack of organisation prepared to face an emergency such as COVID-19, partly explains the differences observed.29 Besides, in the first wave, hospital resources (such as ICU beds, number of non-invasive ventilators or high-flow nasal oxygen therapy devices) and human resources were not sufficient to cope with the high demand for medical care.30 ICU capacity is known to be an important indicator of hospital stress (health system resilience) which is associated with a reduction in quality of care and poorer patient outcomes.31 In addition, other factors such as the increasing knowledge about the disease, facilitated by the rapid publication of clinical trials analysing new treatments,8 or the impact of public health surveillance measures, such as lockdowns,32 could explain this reduction in mortality. The harvest effect could also explain this decrease in mortality, as deaths that would have occurred anyway in subsequent waves may have been precipitated by the high mortality in the first wave of COVID-19.33 Similarly, the aggressiveness of SARS-CoV-2 varied between strains and may also have played a role in the reduction in mortality.34
As expected, mortality was higher among patients with ceiling care. In this group of patients, there are no differences in mortality in the first three waves, but there is a decrease in mortality in wave 4 (OR 0.38, 95% CI 0.25 to 0.58) (figure 3B). In Spain, this fourth wave mainly affected young patients. Older patients, who were more likely to be assigned a ceiling of care, were already vaccinated at that time.35 A study in nursing homes in our geographical area (Catalonia)36 showed that vaccination was associated with a 95% reduction in mortality among nursing home residents. Studies in Italy and Switzerland also showed that the vaccine was about 95% effective against death in the general population.33 37 These results therefore suggest that there is no improvement in medical management that affects in-hospital mortality until wave 4, which coincides with the elderly vaccination campaign. The lack of a contrafactual scenario in which people received intensive care makes it difficult to assess any potential benefit. Further research on this topic and replication of these results in other cohorts would be needed. Moreover, it will be of interest to study the management of ceiling of care in other cultural settings. It would also be interesting to investigate whether the impact of ceiling of care is the same on other outcomes, such as complications or length of hospital stay.
The high probability of a new epidemic caused by an infectious organism merits in-depth reflection by the medical and scientific community, in particular to reach a consensus on the definition of ceiling of care and to define a guideline for the management of patients who are candidates for a ceiling of care.38 In the event of a future pandemic caused by an infectious organism, the challenge will be to improve mortality in patients with ceiling of care. To this end, the scientific community needs to develop an action plan that will enable a rapid response in terms of both human resources (by increasing the number of trained health workers), and facilities (eg, so that the ICUs can quickly increase the number of beds).39
Our study has some limitations that should be acknowledged. Excluding subjects who were discharged or died within 24 hours of admission may introduce selection bias by systematically omitting individuals with atypically short hospital stays. This limitation should be considered when interpreting the generalisability of the study findings. Moreover, we could have residual confounding because even after using all the characteristics available at admission to make the baseline status of patients comparable, there may be unobserved characteristics that make patients different between waves. For example, we knew whether a patient had pathology or not, but we could not take into account how advanced it was. A variable that collects information on patients’ frailty at baseline might also be of interest for a better risk assessment. In addition, vaccines and treatments could not be used in the matching: vaccines because they did not exist in the first wave10 and treatments because they changed drastically between waves due to increasing knowledge about the disease.7 8 Moreover, we do not have data on the follow-up of patients with regard to treatments received during hospitalisation, which could help to understand some of the differences in mortality. Another limitation of the study is that we assumed that the missing values in our data were at random and imputed them using standard techniques. To account for this, a sensitivity analysis was performed repeating the analysis only with patients who had complete information on all variables, and the results were in the same line, confirming the robustness of the analysis. Moreover, we cannot guarantee that the same criteria were used to define the therapeutic ceiling of care in all hospitals. In fact, one of the challenges in clinical practice during the COVID-19 pandemic was to define the ceiling of care for infected patients. Even though the definition of ceiling of care is not a standardised one, the definition in the MetroSud cohort was a pragmatic one which would be readable and understood by clinician teams involved in reaching these decisions. Our definition is consistent with that used in the Leeds cohort13 and with the one used in a multicentre study to identify factors influencing ceiling of treatment in an emergency department.40 In addition, when the study protocol was written, little was known about COVID-19, including the lack of immunity and the possibility of reinfection. Before the emergence of the Omicron strain, the incidence of COVID-19 reinfection leading to hospitalisation was very low (<1%).41 Our last wave included patients from July to August 2021, when the Omicron strain had not yet reached Spain and the incidence of reinfection was still very low. However, we could not rule out the possibility that some subjects from the third or fourth waves had previously been included in the study. Due to data protection laws, we did not have access to patients’ clinical records, which prevented verification. Nevertheless, participating physicians were aware of this possibility and took measures to avoid case duplication.
The strengths of our study are the large number of subjects included from different hospitals and from four different waves of the pandemic, and the availability of information on ceiling of care. In addition, the different methods used to compare in-hospital mortality by waves led to the same results, demonstrating the robustness of the analysis.
In conclusion, knowing that the evolution of in-hospital mortality through waves is different in patients with and without ceiling of care could help the scientific community to address the management of patients with ceiling of care to improve their outcomes in a new pandemic scenario. The lessons learnt from the COVID-19 pandemic could help healthcare professionals and health policy-makers to face future pandemics.
Data availability statement
Data are available upon reasonable request. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. The study was approved by the Bellvitge Hospital Research Ethics Committee with medicines (CREm), with reference PR140/20 and code HUB-INF-COHORT·HUB·COVID, in accordance with Spanish legislation and was performed in accordance with the Helsinki Declaration of 1964. The need for patient informed consent was waived by the ethics committee. Bellvitge’s CREm decision was the basis for the approval of the remaining hospital centres.
Acknowledgments
We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Footnotes
X @npallaresf
Collaborators MetroSud Study group: Gabriela Abelenda-Alonso, Alexander Rombauts, Isabel Oriol, Antonella F. Simonetti, Alejandro Rodríguez-Molinero, Elisenda Izquierdo, Vicens Díaz-Brito, Carlota Gudiol, Judit Aranda-Lobo, Marta Arroyo, Carlos Pérez-López, Montserrat Sanmartí, Encarna Moreno, Maria C Alvarez, Ana Faura, Martha González, Paula Cruz, Mireia Colom, Andrea Perez, Laura Serrano. DIVINE Study group: Mireia Besalú, Erik Cobo, Jordi Cortés, Daniel Fernández, Leire Garmendia, Guadalupe Gómez, Pilar Hereu, Klaus Langohr, Gemma Molist, Núria Pérez-Álvarez, Xavier Piulachs.
Contributors Conceptual design was performed by SV, JC, CT and NP. MetroSud cohort data were provided by SV and JC. Statistical analysis was performed by CT and NP. The first draft of the manuscript was written by NP and revised by JC and CT. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript. CT is the guarantor.
Funding This work was partially funded by Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya (2020PANDE00148). The funding was used to collect data from the 4th wave. This work has also been supported by grant 2021 SGR 01421 (GRBIO) administrated by the Departament de Recerca i Universitats de la Generalitat de Catalunya (Spain).
Competing interests CT has received fees for speaker lectures and talks from Gedeon Richter, outside the submitted work. The rest of authors declare that they have no competing interests.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.