Article Text
Abstract
Background A novel automated system for the control of the inspired fraction of oxygen, named LeoClac, has been implemented on a mechanical ventilator. The system uses a separate sensor for the measurement of peripheral oxygen saturation which is connected directly to the ventilator. We hypothesise that LeoClac will be superior to manual control in keeping critically ill and mechanically ventilated patients in a SpO2-target range (93–96%).
Methods This is a randomised controlled, single-centre superiority study with two parallel groups including 40 patients. Mechanically ventilated patients treated on the intensive care unit (ICU) will be screened for eligibility and included in the study after written informed consent. Patients in the intervention group will be treated with LeoClac. In the control group, FiO2 will be controlled manually by the intensive care team. The primary endpoint of the study is the proportion of time in the target zone for peripheral oxygen saturation within the first 24 hours following randomisation. Secondary endpoints include the analysis of hyperoxia and hypoxia, number of changes in FiO2, number and reasons for self-aborts and manual overrides of the automated system, proportion of time in target zone for peripheral oxygen saturation in the subgroups of patients with hypoxemic respiratory failure and acute hypercapnic respiratory failure. Furthermore, ventilator-free days and ICU mortality at day 28 will be analysed.
Analysis The precise control of FiO2 with the aim of avoiding both hyperoxia and hypoxia is a fundamental challenge in the highly technical field of mechanical ventilation. Incorporation of patient heterogeneity, the benefits of reduced manual intervention and the potential to optimise treatment outcomes underscore the importance of this research. By addressing the complexities of precise oxygen control in adults, this study contributes to the advancement of critical care practices and may improve patient outcomes.
Ethics The study protocol was approved by the ethics committee of the Christian-Albrechts-University Kiel, Germany, on 17 May 2023.
Trial registration number DRKS00032113.
- ANAESTHETICS
- Ventilators, Mechanical
- Oxygen Saturation
- INTENSIVE & CRITICAL CARE
- Lung Diseases
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study aims to evaluate whether an automated system can effectively optimise oxygen therapy in critically ill, invasively ventilated patients.
This study plans to investigate the benefits and limitations of automated oxygen control in a very heterogeneous sample of adult intensive care patients.
The novel system will be used for up to 28 days per patient to evaluate the feasibility of the sensors in daily routine.
Introduction
Background and rationale
Oxygen therapy plays a crucial role in the therapy of critically ill patients, ensuring adequate oxygen delivery while avoiding hypoxia and hyperoxia. Nevertheless, overdosing oxygen may be harmful, as both excessively high and low arterial partial pressure of oxygen (PaO2) are associated with increased mortality.1 2 Stolmeijer et al indicated that liberal oxygen therapy leads to hyperoxia and may also affect survival.3 A recent meta-analysis also highlighted the risks of liberal oxygen therapy, showing increased mortality compared with conservative oxygen therapy.4 The current German guideline recommends titrating peripheral oxygen saturation (SpO2) between 92% and 96% in mechanically ventilated patients.5 In adult patients, sensitivity to oxygen is not as pronounced; yet, neither the consequences of hypoxia or hyperoxia nor the associated economic follow-up costs should be underestimated.6–8
Currently, manual oxygen (FiO2) control based on SpO2 and PaO2 measurements is the standard in clinical practice. However, based on neonatal studies, compliance to peripheral oxygen targets is poor.9 10 This finding might also be applicable to adults and carries the risk of unrecognised hypoxia or hyperoxia. Additionally, healthcare professionals often prioritise avoiding desaturation over avoiding hyperoxia, potentially leading to oxygen oversupply.11 Therefore, it is crucial to carefully monitor patients’ response to therapy and adjust oxygen delivery accordingly.
To address the limitations of manual control, an automated system for FiO2 control would be desirable. Its aim is to continuously modulate oxygen supply, ensure normoxemia and prevent desaturation. In a study by Lellouche et al, automated oxygen flow titration was superior to constant oxygen flow in maintaining SpO2 levels.12 Saihi et al demonstrated that an automated FiO2 controller, based on continuous oxygen saturation, was capable of maintaining SpO2 reliably within a predefined target range.13 Intellivent-ASV (fully automated closed loop ventilation) has been the sole available ventilation mode including automated FiO2 control in invasively ventilated adult patients. The algorithm in Intellivent-ASV compares SpO2 values with the target range and automatically adjusts the FiO2 to maintain desired saturation levels.11 However, access to Intellivent-ASV is limited, available only on selected ventilators. A new alternative, the Loewenstein closed-loop automatic oxygen control (LeoClac), offers rapid modulation of FiO2 based on a separate SpO2 measurement attached to the ventilator. The user sets a target SpO2 range to be maintained before starting the FiO2 control. The user also defines a FiO2 threshold for receiving an alarm. An alarm is triggered if the FiO2 alarm threshold is exceeded; however, the automatic regulation continues until deactivation by the user (‘manual override’). The SpO2 measurement is based on a configurable number of pulse waves. FiO2 reductions occur every 2 minutes, increases are limited to every 45 seconds to avoid ‘swinging’ of the system.
Recent publications suggest that closed-loop control devices maintain higher saturation levels, spend less time below the target saturation and save oxygen resources.11 14 15 Moreover, an automated system may contribute to maintaining sufficient oxygenation during exercise and physiotherapy when oxygen consumption rises.16
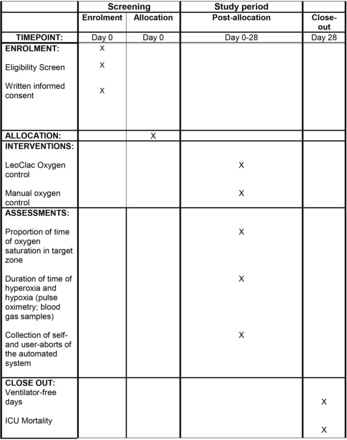
While automated closed-loop systems have been well evaluated in infants to prevent hyperoxia and retinal damage, long-term evaluation in adults is lacking.11 17 18 However, Bialais et al were able to demonstrate that Intellivent-ASV provides safe ventilation with optimised oxygenation and reduced workload on caregivers over a time period of 48 hours.19 Nevertheless, the aim of this study is to further investigate the benefits and limitations of automated control of inspired FiO2 in invasively ventilated critically ill adults. The SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) figure provides an overview of the phases of the trial and data collection timepoints (figure 1). The primary endpoint of this trial is the proportion of time in the target zone for peripheral oxygen saturation within the first 24 hours following randomisation, calculated based on all usable time with a valid SpO2 signal. This also includes the analysis of blood gas samples, self-aborts and manual overrides of the automated system and the number of changes in FiO2. Furthermore, the proportion of time in the peripheral oxygen target zone in patients with acute hypoxaemic respiratory failure and acute hypercapnic respiratory failure will be further analysed.
The SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) figure provides an overview of the different phases of the trial and outlines the data collection timepoints. ICU, intensive care unit.
Explanation for the choice of comparators
The primary objective is to examine the compliance with the target zone for peripheral oxygen saturation (SpO2) using the LeoClac automated system compared with manual control. Thus, the comparators in this trial are manual and automated FiO2 control. Manual control refers to the standard practice of manually adjusting the inspired fraction of oxygen (FiO2) based on clinical judgement. Periodic assessments of the patient’s condition and reference to current and recent SpO2 levels. It represents the current approach used in clinical practice.
The use of manual control as a comparator allows for a direct comparison with the LeoClac automated system. By assessing the efficacy of LeoClac in maintaining SpO2 within the target zone, it can be determined whether the automated system provides improved control and adherence to the desired oxygen saturation levels compared with standard manual control methods.
By comparing the performance of the LeoClac system to manual control, the trial aims to evaluate whether the automated system can effectively optimise oxygen therapy and improve patient outcomes in critically ill, invasively ventilated patients.
Objectives
The primary objective of this study is to investigate compliance within the predefined SpO2 target zone of 93% to 96% in critically ill and invasively ventilated patients comparing the novel ‘LeoClac’ controller to manual control.
Trial design
This trial is a randomised, controlled, single-centre superiority trial with two parallel groups. The study aims to assess the proportion of time spent in the target zone for peripheral oxygen saturation within the first 24 hours following randomisation as the primary endpoint. Randomisation is conducted using random permuted block randomisation with a 1:1 allocation ratio. Blinding is not feasible in this study.
Methods: participants, interventions and outcomes
Study setting
The study will be performed on all interdisciplinary surgical intensive care units of the Department of Anesthesiology and Intensive Care Medicine, University Medical Centre Schleswig-Holstein, Campus Kiel.
Eligibility criteria
To be considered eligible for the study, patients must meet the following inclusion criteria: (1) Intubated or tracheotomised patients requiring mechanical ventilation for a duration of at least 9 hours as of 09:00 am, (2) participants must be at least 18 years old and written informed consent must be obtained. Potential patients will be excluded from the study if any of the following exclusion criteria are met: (1) Inability to measure peripheral oxygen saturation, (2) absence of a detectable pulsatile plethysmography curve, (3) clinical indication for hyperoxia (SpO2 target >96%), (4) expected extubation within the next 24 hours, (5) negative presumed will regarding study participation or (6) known pregnancy.
Patient and public involvement
Patients or the public are not involved in the design, or conduct, or reporting or dissemination plans of our research.
Intervention description
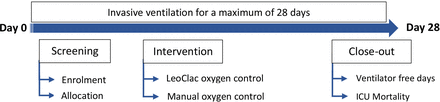
After obtaining written informed consent, patients will be included in the study. Patients randomised to the intervention group will be mechanically ventilated with an automated control of the inspired oxygen fraction. Therefore, the ventilator will automatically adjust the amount of oxygen required based on oxygen saturation, with the goal of always maintaining oxygen saturation between 93% and 96%. Regardless of group assignment, all patients receive an additional oxygen sensor as part of the LeoClac system. If the two oxygen saturation sensors show different values, the measurements from the LeoClac device should be used. In case of uncertainty, an arterial blood gas analysis should be performed for verification. LeoClac will be used from the start of the study period until the end of invasive ventilation (extubation, decannulation, dismission or death) or day 28. Figure 2 shows the participant timeline and provides an overview of screening, intervention and close-out.
The participant timeline provides an overview of screening, intervention and close-out. ICU, intensive care unit.
Criteria for discontinuing or modifying allocated interventions
If instances of uncontrollable respiratory instability occur during the intervention, the intervention will be discontinued. Treating physicians (who are not part of the study team) can suspend the study at any time. LeoClac can be deactivated at any time and for any reason. The reason will be documented in the case report form. Furthermore, participants/representatives may withdraw from the study without citing a reason at any time. There is no provision for modifying the assigned intervention.
Strategies to improve adherence to interventions
Adherence to the study protocol is ensured by staff training courses and information leaflets, which are attached to the patient’s ventilator.
Relevant concomitant care permitted or prohibited during the trial
All procedures and interventions in this study adhere to established internal standard procedures required for optimal patient treatment.
Outcomes
The primary endpoint of this trial is the proportion of time in the target zone for peripheral oxygen saturation within the first 24 h hours following randomisation.
Secondary endpoints apply to the entire study period and include the following:
Proportion of time with automated control of inspired fraction of oxygen activated.
Proportion of time with hypoxia according to pulse oximetry (SpO2<90%).
Proportion of time with hyperoxia according to pulse oximetry (SpO2 >98%).
Number of blood gas samples with hypoxia (n/day; PaO2 <60 mm Hg).
Number of blood gas samples with hyperoxia (n/day; PaO2 >110 mm Hg).
Number of changes of inspired fraction of oxygen (n/day).
Number of and reasons for self-aborts of the automated system (n/day).
Number of and reasons for manual overrides of the automated system (n/day).
Proportion of time in target zone for peripheral oxygen saturation in the subgroup of patients with acute hypoxaemic respiratory failure.
Proportion of time in the target zone for peripheral oxygen saturation in the subgroup of patients with acute hypercapnic respiratory failure.
Ventilator-free days alive at day 28.
Intensive care unit (ICU) mortality within 28 days.
Participant timeline
Figure 2 shows the participant timeline.
Sample size
Based on data with other automated systems and on our clinical experience with manual oxygen control, we expect a time within SpO2 target range of 80% (SD 20) with LeoClac and of 60% (SD 20) with manual control. For a two-sided Mann-Whitney U test, power calculation with 1-Beta=0.8 and alpha 0.05 yields a required sample size of 2×20 patients to be sufficient.
Recruitment
Eligible patients will be screened for study participation every morning (Monday–Friday). Screening will be conducted by senior physicians. Recruitment will be continuously conducted by physicians of the research team until the target randomised sample size of 40 participants is achieved. Based on current clinical case numbers, this will take approximately 6 months.
Assignment of interventions: allocation
Sequence generation
Randomisation is performed by random permuted block randomisation with a 1:1 allocation ratio, minimum block size: 4; maximum block size: 8; increment: 2. The randomisation is performed electronically using www.studyrandomizer.com.
Concealment mechanism
The randomisation is performed electronically using www.studyrandomizer.com.
Implementation
The allocation sequence is generated electronically.
Who will be blinded
Blinding of the study team is not feasible due to the study concept. Patients and their representatives will be blinded to the intervention.
Data collection and management
Plans for assessment and collection of outcomes
Demographic and clinical data are recorded on electronic-based case report forms (eCRFs) by data collectors. Ventilator measurement data and oxygen saturation values are exported electronically from the ventilator and the monitoring unit. To determine the primary and further secondary outcomes, ventilator data and monitoring data will be analysed by the data analysing team as described in the statistical methods for primary and secondary outcomes section.
Plans to promote participant retention and complete follow-up
No follow-up for clinically relevant outcome measurement needed.
Data management
All participant information will be stored on password-protected databases to which only research team members have access. Collected data are pseudonymised by a coded ID (identification) number. All records that contain names or other personal identifiers, such as informed consent forms, will be stored separately from study records. Demographic and clinical data will be stored on eCRFs. Log files containing ventilator data are exported from ventilators and saved on a password-secured network drive.
Statistical methods for primary and secondary outcomes
Descriptive statistical analyses (mean±SD, median and 95% CI, where appropriate) will be used. As we expect the proportion of time in the target zone for peripheral oxygen saturation within the first 24 hours following randomisation to be non-normally distributed, the primary endpoint will be compared between study groups with a Mann-Whitney U test. Categorical endpoints will be investigated using Fisher’s exact test. Other between-group comparisons of numerical endpoints will be conducted using a two-sided t-test or Mann-Whitney U test, as appropriate. Testing for normal distribution will be performed with the Shapiro-Wilk test.
Methods for additional analyses (eg, subgroup analyses)
Not applicable, no further analyses planned.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data
Datasets will be excluded if the study intervention was not performed, or if the primary endpoint cannot be evaluated.
Methods: monitoring
Composition of the data monitoring committee, its role and reporting structure
As no interim analyses are planned or will be performed, a data monitoring committee has not been organised for this study.
Interim analyses
Not applicable, there will be no interim analyses.
Adverse event reporting and harms
Adverse events and other unintended effects of the trial will be collected, assessed and immediately reported to the principal investigator.
Frequency and plans for auditing trial conduct
Not applicable, an auditing trail conduct is not required.
Ethics and dissemination
Ethics approval and consent to participate
The local review board of the Medical Faculty of the Christian-Albrecht University approved the study, reference number D 449/22. The patient information has been included in the online supplemental file S1. Written informed consent to participate will be obtained from all patients.
Supplemental material
Plans for communicating important protocol amendments to relevant parties (eg, trial participants, ethical committees)
Any changes in protocol will be submitted to the local ethics committee for approval, all changes will be communicated to the study team via email and during the regular study group meetings. The trial record on the German Clinical Trials Register (DRKS) will be updated accordingly.
Who will take informed consent?
In this randomised controlled trial investigating a novel system for automated FO2 control in critically ill, mechanically ventilated patients, the process of obtaining informed consent will be multistep based due to the patients’ critical condition. Since most patients will be unable to provide written consent themselves, the patient’s presumed will regarding study participation will be determined in consultation with their relatives or representatives. Written informed consent will then be obtained from the patient’s legal representatives prior to their participation in the study.
Patients will be assessed for potential study inclusion daily (Monday–Friday). As soon as a patient regains consciousness, their written informed consent will be sought. It is important to note that in accordance with the principles outlined in the Declaration of Helsinki and national regulations, consent can be withdrawn at any time, without the need to specify reasons, and without compromising the patient’s future medical care.
Additional consent provisions for collection and use of participant data and biological specimens
No additional data will be collected.
Confidentiality
Data are handled confidentially, and the storage of patient-related medical data is pseudonymised. No features are transferred that allow direct identification of specific participants. The subject identification code list to personal data is accessible only to the principal investigator. All further records containing names or other personal identifiers, such as informed consent forms, are kept separate from the study data identified by code number. Data collection, coding, security and storage will comply with the provisions of the German Federal Data Protection Act (BDSG) and the EU General Data Protection Regulation (EU-GDPR). Accordingly, records and documents related to the clinical trial will be kept for at least 15 years.
Provisions for post-trial care
After completion of the study, patients will continue to receive care in our ICU until they reach a level of recovery that allows for transfer to a general ward or a rehabilitation clinic. Patients are encouraged to contact our clinic at any time if they have any concerns.
Dissemination plans
Once the study has been completed, the results will be published in a peer-reviewed scientific journal and presented on national and international conferences for anaesthesiology and intensive care medicine.
Plans to give access to the full protocol, participant level data and statistical code
Datasets containing anonymised patient data will be made accessible on reasonable request to the corresponding author, in compliance with European data protection regulations (EU-GDPR).
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use
No biological specimens will be collected.
Discussion
This study aims to evaluate the efficacy of an automated closed-loop oxygen control system to examine compliance with the SpO2 target zone in critically ill and invasively ventilated adult patients. The precise control of oxygen demand with the aim of avoiding both hyperoxia and hypoxia is a central challenge in the highly technical field of mechanical ventilation. From the paediatric and neonatal point of view, automatic oxygen control has already been able to demonstrate significant success in numerous studies.17 20–22 With the novel LeoClac function, the oxygen demand is continuously monitored and checked several times per minute. Increases in oxygen demand are therefore immediately detected, for instance, when the patient is more active or mobilised. This real-time adaptability may significantly reduce the likelihood of sudden fluctuations in oxygen saturations.
It is imperative to acknowledge the patient heterogeneity observed in critically ill patients. Understanding how different patients respond to automated oxygen control is vital for tailoring treatment strategies to individual needs. The reduction of manual intervention could become a significant advantage of automated oxygen control. The current standard often requires manual adjustment and monitoring of oxygen supply, placing a significant burden on medical staff, especially in patients with high oxygen demand. The automation of this process holds promise, especially in high-workload situations or when multiple patients are being cared for simultaneously, where the system can increase efficiency and reduce the burden on medical staff. In addition, the precise and controlled delivery of oxygen holds the potential to optimise treatment outcomes.
Incorporation of patient heterogeneity, the benefits of reduced manual intervention and the potential to optimise treatment outcomes underscore the paramount importance of this research. By addressing the complexities of precise oxygen control in adults, this study contributes to the advancement of critical care practices and may improve patient outcomes.
Trial status
The study protocol was approved by the ethics committee of the Christian-Albrechts-University Kiel, Germany, on 1 February 2023. Recruitment has begun in December 2023 and is expected to end in June 2025.
Ethics statements
Patient consent for publication
Footnotes
Contributors All authors were involved in the design of the study and have contributed and approved publication of the study protocol. CE: Formal analysis, writing and editing the manuscript. HS: Data acquisition, writing and editing the manuscript. LH: Screening of patients, data acquisition, reviewing of the manuscript. AS: Screening of patients, data acquisition, reviewing of the manuscript. ML gave important intellectual input and critically reviewed the study protocol. TB: Conceived the LeoClac controller, drafted and reviewed the study protocol, screening of patients, data acquisition. DS: Acted as guarantor, original idea for study, first draft of study protocol, principal investigator. All authors read and approved the final manuscript. All authors contributed to the study according to the criteria of the ICMJE. ChatGPT was used to improve language and grammar to reach a linguistic level adequate for publication. Using ChatGPT was used to enhance the language, grammar and sentence structure of the original text. However, it is important to note that the core content and meaning of the text remained unchanged and all changes from ChatGPT were reviewed by the authors.
Funding Löwenstein Medical provided single-use peripheral oxygen sensors free of charge during the study period. No further funding has been received.
Competing interests We have received study material (SpO2 sensors) from Löwenstein Medical Innovation, Steinbach, Germany. TB and DS received grants from the Federal Ministry of Education and Research. TB received consulting and lecture fees (Löwenstein Medical, Vyaire Medical). DS received lecture fees (Sedana, Aerogen, Löwenstein). All other authors have no competing interests to declare.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.