Article Text
Abstract
Objective Drug-induced ischaemic colitis is a significant adverse event (AE) in clinical practice. This study aimed to recognise the top drugs associated with the risk of ischaemic colitis based on the FDA Adverse Event Reporting System (FAERS) database.
Design A cross-sectional design.
Setting All data retrieved from the FAERS database from the first quarter of 2004 to the fourth quarter of 2023.
Participants A total of 5664 drug-induced ischaemic colitis AEs eligible for screening.
Primary and secondary outcome measures The Medical Dictionary for Regulatory Activities was used to identify ischaemic colitis (code: 10009895) cases. Disproportionality analysis for drug-associated ischaemic colitis signals.
Results Drug-induced ischaemic colitis AEs were more prevalent in females (60.12%) and individuals aged ≥65 years (34.25%). The common outcomes were hospitalisation (46.85%) and death (9.73%). Disproportionality analysis identified 91 ischaemic colitis signals and the top 30 drugs mainly involved in the gastrointestinal and nervous systems. The top five drugs with the highest reported OR, proportional reporting ratio, information component and the empirical Bayesian geometric mean, were alosetron, tegaserod, osmoprep, naratriptan and kayexalate. Additionally, 20 of the top 30 drugs did not have ischaemic colitis risk indicated in the package insert.
Conclusions This study identified key drugs associated with ischaemic colitis, particularly alosetron, tegaserod, osmoprep, naratriptan and kayexalate. Notably, two-thirds of these drugs lacked ischaemic colitis warnings in their package inserts. These findings underscore the need for greater clinical vigilance, improved regulatory oversight and further research to clarify underlying mechanisms and support safer medication use.
- Adverse events
- Drug Utilization
- Gastroduodenal disease
Data availability statement
Data are available in a public, open access repository.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study used the FDA Adverse Event Reporting System (FAERS), a large and real-world pharmacovigilance database, to investigate drug-induced ischaemic colitis.
Disproportionality analysis methods, including reported OR, proportional reporting ratio, Bayesian confidence propagation neural network and empirical Bayesian geometric mean, were used to evaluate the associations between drugs and adverse events.
The use of a cross-sectional design limits the ability to infer causality between drug use and ischaemic colitis.
FAERS is a self-reporting system, which may introduce biases such as under-reporting, incomplete information, and variability in data quality.
Introduction
Ischaemic colitis is the most frequent type of colonic vascular injury, accounting for approximately 50%–60% of ischaemic disorders of the gastrointestinal tract.1 2 The incidence of ischaemic colitis increases with the ageing population, primarily affecting the elderly.3 The aetiology of ischaemic colitis is either physiological, including hypotension, secondary to embolism/thrombosis or iatrogenic, involving secondary to drugs and surgery.4 Clinically, ischaemic colitis mainly presents with abdominal pain, diarrhoea and rectal bleeding.5 Decreased blood flow in ischaemic colitis can lead to a spectrum of lesions, ranging from focal ischaemia to severe segmental intestinal infarction.4 6 In addition, ischaemic colitis can cause complications, including obstruction, necrosis and perforation. Therefore, identification of patients with ischaemic colitis is recommended, with colonoscopic evaluation ideally performed within 48 hours of symptom onset.4 7 Colonoscopy can differentiate cases amenable to conservative treatment from those requiring urgent resection.
Ischaemic colitis can occur secondary to various conditions such as mesenteric artery embolism, thrombosis or trauma, which may lead to occlusive vascular diseases and compromised colon perfusion, as well as hypoperfusion states resulting from congestive heart failure. Additionally, a myriad of drugs predispose to ischaemic colitis, including antibiotics (amoxicillin-clavulanate),8 anorectic agents (fenfluramine), chemotherapeutic agents, constipation-inducing drugs (loperamide),9 decongestants (pseudoephedrine),10 cardiac glycosides, diuretics, ergot alkaloids, hormonal therapies, statins, illicit drugs, tumour necrosis factor-alpha inhibitors,11 laxatives (lactulose, bisacodyl),12 non-steroidal anti-inflammatory drugs (NSAIDs), psychotropic medications (amphetamine, quetiapine)13 and serotonin agonists/antagonists.14 15 Currently, information on ischaemic colitis risks is primarily documented in package inserts, depending on the results of clinical trials. Due to constraints in sample size and follow-up duration, clinical trials may not precisely reflect the occurrence of drug-induced ischaemic colitis in real-world situations. The ischaemic colitis risk of certain drugs may not be readily recognised in clinical trials. In addition, the majority of drug-induced ischaemic colitis in the clinical setting was reported in the form of case reports, and data from large-scale real-world studies are not available. Therefore, the correlation between drugs and ischaemic colitis still needs to be completed.
Postmarketing surveillance is a vital method to determine the association between drugs and adverse events (AEs). FDA Adverse Event Reporting System (FAERS) is a self-reporting system for collecting postmarketing AEs for drugs. FAERS, owing to its extensive data repository and open accessibility, is commonly used in drug signal mining studies.16 This study aimed to investigate the risk of drug-induced ischaemic colitis by the FAERS database thoroughly and to identify drugs with a potential risk of ischaemic colitis that are not described in the drug inserts.
Methods
Data sources
The data for this study were obtained from the FAERS, covering the period from the first quarter of 2004 to the fourth quarter of 2023. The American Standard Code for Information Interchange report files were downloaded directly from the FAERS Public Dashboard. They included the following datasets: DEMO (demographic information), DRUG (drug information), REAC (reaction information) and OUTC (outcome information). The data were imported into MySQL V.15.0 for structured storage and efficient query management. Navicat Premium V.15 software was employed for database management and data retrieval. Before analysis, data cleaning and standardisation were performed to ensure consistency and accuracy.
Definition of AEs, drugs and outcomes
AEs extracted from the FAERS database were coded using the preferred term (PT) system in the Medical Dictionary for Regulatory Activities (MedDRA) to standardise nomenclature. To identify cases of drug-induced colitis ischaemic, reports containing the PT ‘colitis ischaemic’ (MedDRA code: 10009895) in the REAC dataset were retrieved. Once these reports were identified, all associated drug records were extracted for further analysis. Since FAERS reports may include both generic drug names and brand names, standardising drug nomenclature was essential to maintain consistency in analysis. Drug names were first matched to their corresponding generic names using the DrugBank database, which serves as a comprehensive reference for drug classification. Any drug names that could not be retrieved from the DrugBank database were considered incorrect reports and were subjected to manual elimination. This standardisation process helped ensure data accuracy and reliability for statistical evaluation. In this study, outcomes are categorised into seven types. Disability refers to cases in which the AE resulted in a substantial disruption of a person’s ability to conduct normal life functions. Required intervention to prevent permanent impairment/damage refers to cases where medical or surgical intervention was deemed necessary to preclude permanent impairment of body function or prevent permanent damage to body structure. Life-threatening refers to cases where the patient was at substantial risk of dying at the time of the AE or where continued use of the medical product might have resulted in death.
Statistical analysis
Descriptive analysis was conducted to describe the clinical characteristics of patients with drug-induced ischaemic colitis, including age, gender, reporter, outcome (hospitalisation, death, life threatening, disability, required intervention to prevent permanent impairment/damage, congenital anomaly, other serious) and reported country. Individual safety reports (ISRs) were enumerated, with each ISR considered an AE report. To identify potential associations between drugs and ischaemic colitis, disproportionality analysis was employed as a hypothesis-generating approach. The top 30 drugs most strongly associated with ischaemic colitis were identified based on their disproportionality metrics. Four widely used disproportionality analysis methods were applied to detect potential drug-AE signals. All algorithms rely on a 2×2 contingency table (table 1). Specific formulas and cut-off thresholds were detailed as follows, and statistical analyses were performed using R software. A higher value indicates a stronger statistical relationship between the suspect drug and suspect AE.
Four grid table
Reported OR (ROR) formula: 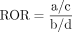 ,
, 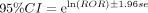
Signal criteria: ROR≥3, a ≥3 and the lower limit of the 95% CI >1.
Proportional reporting ratio (PRR) Formula:  ,
, 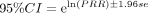
Signal criteria: PRR ≥2, a ≥3 and the lower limit of the 95% CI >1.
Bayesian confidence propagation neural network (BCPNN) formula:
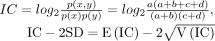
Signal criteria: the lower bound of the 95% CI (IC025) >0.
Empirical Bayesian geometric mean (EBGM) formula: 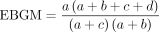 ,
, 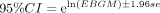
Signal criteria: EBGM05>2 (EBGM05 denotes the lower bound of the 95% CI).
In this study, we employed ROR, PRR, BCPNN and EBGM to detect drug-AE signals, considering the unique strengths of each method to ensure a more comprehensive and reliable signal detection process: ROR corrects for biases caused by a small number of reports for specific events. PRR offers higher specificity than ROR, reducing the likelihood of false positives. BCPNN integrates multisource data and performs cross-validation, enhancing robustness. EBGM adjusts for variability through Bayesian modelling, making it particularly effective for detecting rare AEs. By combining these methods, we leveraged their respective advantages to broaden the detection scope, validate findings from multiple perspectives and improve the accuracy and reliability of safety signal detection. The joint application of multiple algorithms allows for cross-validation, reducing false positives and improving the detection of rare adverse reactions.
Patient and public involvement
None.
Results
Descriptive analysis
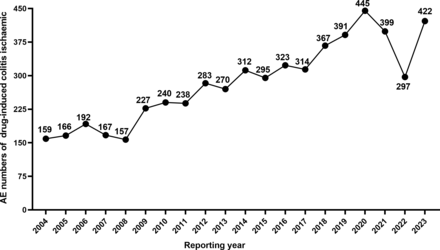
A total of 5664 drug-induced ischaemic colitis AEs were reported in the FAERS database from the first quarter of 2004 to the fourth quarter of 2023. As shown in figure 1, the number of reported AEs of drug-induced ischaemic colitis peaked in 2020 at 445 cases. Beginning in 2021, the number of AEs begins to decline, but the overall trend exhibits increasing volatility from 2004 to 2023.
Number of reported AEs of drug-induced ischaemic colitis from 2004 to 2023. AE, adverse event.
The clinical characteristics of these 5664 AE reports are listed in table 2. Ischaemic colitis was more prevalent in females (60.12%) than in males (31.02%). Ischaemic colitis was more likely to occur in ≥65 years (34.25%), followed by 41–64 years of age (31.48%), 19–40 years of age (10.52%) and ≤18 years (1.36%). These AE reports from physician reporters had the highest percentage (44.26%), thus increasing the credibility of this study. The top five most frequently reported outcomes were hospitalisation (46.85%), followed by death (9.73%), life-threatening (6.28%), disability (2.00%) and required intervention to prevent permanent impairment (0.49%). Notably, the most number of drug-induced ischaemic colitis reports were from the USA (n=1534, 27.08%), followed by Japan (n=657, 11.60%), France (n=464, 8.19%) and the UK (n=210, 3.71%). Additionally, the most frequently reported time-to-onset of drug-induced ischaemic colitis was ≥60 days (n=998, 29.10%), followed by <7 days (n=538, 15.69%), 7–28 days (n=361, 10.53%) and 28–60 days (n=223, 6.50%).
Clinical characteristics of reported drug-induced ischaemic colitis
Disproportionality analysis
A total of 91 ischaemic colitis signals were identified according to the ROR ˃3 criteria. The top 30 (ranked by ROR) drugs associated with the highest signal intensity in drug-induced ischaemic colitis are listed in table 3. The top 30 highest drugs used PRR, information component and EBGM methods consistent with the results of RORs. The most common of the top 30 drugs were gastrointestinal and nervous system drugs. These include alosetron (ROR=339.26, 95% CI: 263.31 to 437.11, a serotonin-3 receptor antagonist), tegaserod (ROR=67.52, 95% CI: 55.47 to 82.19, a serotonin-4 receptor antagonist) and eluxadoline (ROR=19.46, 95% CI: 11.28 to 33.59, a μ-opioid receptor agonist) for the treatment of irritable bowel syndrome (IBS). Additionally, drugs for the treatment of constipation and cleansing of the colon in preparation for colonoscopy were included: lubiprostone (ROR=44.03, 95% CI: 27.99 to 69.26), lactulose (ROR=15.34, 95% CI: 5.74 to 40.96), bisacodyl (ROR=33.49, 95% CI: 20.46 to 54.82), suprep bowel prep (ROR=10.63, 95% CI: 5.88 to 19.23), osmoprep (ROR=51.87, 95% CI: 30.59 to 87.97) and prepopik (ROR=16.63, 95% CI: 7.45 to 37.10). Furthermore, serotonin antagonists should be given more attention in drug-induced ischaemic colitis, including 5-HT3 and 5-HT 1B/1D receptor antagonists. Among them, signal strength was granisetron ROR=17.05 (95% CI: 9.43 to 30.86), naratriptan ROR=64.15 (95% CI: 34.31 to 119.94), rizatriptan ROR=33.15 (95% CI: 21.55 to 50.98), zolmitriptan ROR=16.96 (95% CI: 9.11 to 31.59), sumatriptan ROR=14.51 (95% CI: 11.8 to 17.83), respectively. Other neurological drugs that cause drug-induced ischaemic colitis include benztropine mesylate, dexamfetamine, phentermine, and desogestrel and ethinyl estradiol. Moreover, using baloxavir marboxi (ROR=44.95), peramivir (ROR=35.47), oseltamivir (ROR=8.97), ketoprofen (ROR=18.62) and piroxicam (ROR=15.26) for the treatment of influenza and rheumatoid arthritis disease ischaemic colitis should be a concern. Other drugs related to drug-induced ischaemic colitis include daprodustat, ferrlecit, sevelamer carbonate, miglitol, pletal, etelcalcetide and kayexalate. Among the top 30 drugs in drug-induced ischaemic colitis, there were 20 drugs whose instructions do not indicate the risk of ischaemic colitis.
Top 30 drugs for signal strength
Discussion
To the best of our knowledge, this study is the first and largest assessment of drug-induced ischaemic colitis in real world based on the FAERS database. In this study, we outlined the clinical features of these AEs and identified the drugs with the highest associations with drug-induced ischaemic colitis. Many of these drugs are not labelled with ischaemic colitis in the package insert and are not known to present an ischaemic colitis risk.
Age is considered a significant risk factor for ischaemic colitis. A study of 1560 patients with ischaemic colitis showed that 73% were >65 years old, and the incidence increased with age.17 Another study showed that the prevalence of ischaemic colitis was 1.1/100 000 in individuals under 40 years old, while in those over 80.3 years old, the incidence was 107/100 000,18 which suggests that the risk of ischaemic colitis increases with age and may be related to the presence of more cardiovascular and cerebrovascular risk factors in the elderly population. In our study, we also found that drug-induced ischaemic colitis was more common in people ≥65 years of age. Although ischaemic colitis usually occurs in the elderly, reports were suggesting an increasing prevalence of the disease in younger age groups,19 which may be associated with factors such as hypercoagulability, vascular disease, long-distance running, smoking, constipation and contraceptives.20–22 With the exception of gender unknown (8.86%), the percentage of female ischaemic colitis cases identified was 60.12% in our study. In population-based studies, women were more likely than men to suffer ischaemic colitis, with females accounting for 61%–67% of all cases,23 24 which was consistent with our study.
Serotonin receptor antagonists, including 5-HT3, 5-HT4 and 5-HT 1B/1D, should be given enough attention in drug-induced ischaemic colitis. 5-HT(3) antagonists are effective in treating chemotherapy-induced vomiting and diarrhoea, as well as urgency and pain associated with IBS.15 Studies have shown that alosetron, a serotonin-3 (5-HT3) receptor antagonist, was effective in treating diarrhoea, urgency and pain in IBS.25 26 However, it was regrettable that alosetron was withdrawn from the market after 446 000 prescriptions were issued following reports of 49 cases of ischaemic colitis.27 This was an unexpected pharmacological outcome. The mechanism behind this is unclear, as alosetron does not alter colonic blood flow in experimental animals.28 Tegaserod is a serotonin-4 (5-HT4) receptor partial antagonist, with the most common adverse reactions being diarrhoea, headache and abdominal pain. Given that patients with IBS have a higher risk of ischaemic colitis than the general population, no cases of ischaemic colitis associated with tegaserod were reported in over 11 600 patients enrolled in phase III or postmarketing randomised controlled trials.29 This may be because the incidence of ischaemic colitis is low, making it unlikely to be detected in phase III trials. However, there were also studies indicating that the increased incidence of ischaemic colitis caused by tegaserod should be of particular concern.29 30 In the current study, we found the highest number of cases of tegaserod-induced ischaemic colitis (n=103) and a higher risk (ROR=67.52). Although the mechanism needs to be verified, clinical use of tegaserod should be attentive to the risk of ischaemic colitis. In our study, we identified four 5-HT1B/1D receptor agonists that increase the risk of ischaemic colitis: naratriptan, rizatriptan, zolmitriptan and sumatriptan. To our knowledge, there are six reported cases of naratriptan-induced ischaemic colitis, with two cases suggesting a possible association between naratriptan-induced ischaemic colitis and concomitant use of contraceptives.31 Two studies reported the relationship between rizatriptan and ischaemic colitis, with one case report indicating rizatriptan-induced ischaemic colitis and another suggesting that rizatriptan can trigger acute on top of chronic ischaemic colitis.32 33 Nguyen and Lewis found 19 cases of zolmitriptan-induced ischaemic colitis in the FAERS database up to May 2013,34 whereas, in our study, conducted until December 2023, this number increased to 92 cases. Additionally, other studies suggest that zolmitriptan-induced ischaemic colitis may be associated with its overuse35 or vigorous physical activity following zolmitriptan.36 In summary, although we found an increased risk of ischaemic colitis associated with 5-HT1B/1D receptor agonists based on the FAERS database and received support from some case reports, the true incidence of ischaemic colitis induced by 5-HT1B/1D receptor agonists still needs to be accurately determined.
In phase III clinical trials, the most frequent AEs related to eluxadoline were abdominal pain (6.5%), nausea (7.7%) and constipation (8%).37 In March 2017, the Food and Drug Administration (FDA) issued a warning regarding an increased risk of severe pancreatitis in patients receiving eluxadoline treatment without a gallbladder.38 Additionally, a case of ischaemic colitis was reported, with colonoscopy and histological examination revealing colonic ischaemia involving the entire length of colon.39 Our study found the risk of ischaemic colitis associated with eluxadoline to be ROR=19.46. Both lubiprostone and lactulose are medications used to treat constipation. Although they have different mechanisms of action, both are associated with drug-induced ischaemic colitis. The first case of lubiprostone-induced ischaemic colitis was reported in 2013, and symptoms improved on discontinuation of lubiprostone.40 Our study also found that lubiprostone may increase the risk of ischaemic colitis (ROR=44.03). However, other studies have reported that lubiprostone can protect intestinal mucosal barrier function in colitis animal models and improve intestinal barrier function in patients with Crohn’s disease.41 Regarding lactulose, to our knowledge, only two cases of lactulose-induced ischaemic colitis have been reported. Researchers speculate that this may be due to lactulose causing gaseous distension through fermentation by colonic bacteria.42 Bowel cleansers are used for various indications, with the most common being preparation for colonoscopy. Prior to colonoscopy, it is imperative to clear faecal matter from the intestine to effectively visualise abnormalities under the endoscope.43 Bowel cleansing agents constitute a class of medications closely associated with ischaemic colitis.44 We found that bisacodyl, suprep bowel prep (contains sodium sulfate, potassium sulfate and magnesium sulfate), osmoprep (contains sodium phosphate monobasic monohydrate and sodium phosphate dibasic anhydrous. Inert ingredients include polyethylene glycol and magnesium stearate, and prepopik (contains sodium picosulfate, magnesium oxide and anhydrous citric acid) all increase the risk of ischaemic colitis, as documented in their respective package inserts. Therefore, selecting the appropriate bowel cleansers becomes especially crucial in patients with different comorbidities. In addition, in our study, three antiviral drugs and two NSAIDs were found to increase the risk of drug-induced ischaemic colitis. Since influenza A infection itself can induce ischaemic colitis, reports of ischaemic colitis caused by anti-influenza drugs are relatively rare. Kanai et al reported a case of acute ischaemic colitis in a 62-year-old Japanese woman after taking baloxavir marboxil for the treatment of influenza A.45 Regarding oseltamivir, several studies have reported haemorrhagic colitis and ischaemic colitis.46 47 There have been no reports of colitis associated with the anti-influenza drug peramivir. Recent studies suggested that baloxavir marboxil and oseltamivir may induce ischaemic colitis through a shared mechanism, as both drugs exhibit the ability to chelate metal ions in the gastrointestinal tract.48 49 Metal ion homeostasis is crucial for vascular stability, and its disruption may compromise normal blood flow, potentially leading to intestinal ischaemia. Furthermore, drugs that induce constipation represent an additional risk factor for ischaemic colitis, as they can reduce colonic blood flow and increase intraluminal pressure, thereby exacerbating ischaemic conditions. Specifically, unmetabolised baloxavir and its active metabolite have been reported to chelate dietary metal ions within the intestine.45 This chelation process can alter local osmotic balance, thereby increasing the risk of ischaemic colitis.50 These findings highlight the importance of considering the potential vascular effects of metal ion-chelating drugs, particularly in patients receiving antiviral treatment for influenza. Further studies are warranted to elucidate the exact mechanisms underlying these drug-induced vascular alterations and their clinical implications.
Ketoprofen and piroxicam are two common NSAIDs that exert their effects by inhibiting cyclooxygenase-2. NSAIDs are considered to be important triggers for the activation of inflammatory bowel diseases. Yen et al found that long-term use of NSAIDs was independently associated with the development of microscopic colitis.51 Another study indicated that ketoprofen can reduce inflammation and colonic mucosal injury in a rat colitis model, but it was associated with visible gastric bleeding and increased methane output.52 53 The risk of gastrointestinal AEs, including bleeding, ulceration and gastric or intestinal perforation, is reflected in the labels of NSAID drugs, but ischaemic colitis is not mentioned. This should attract more attention in clinical practice.
Our current real-world drug surveillance study provides valuable insights for identifying drugs that may induce ischaemic colitis. However, it should be noted that there are inevitable limitations. First, the lack of detailed case counts for each drug hinders the calculation and comparison of the true incidence rates of ischaemic colitis induced by each drug. Furthermore, the spontaneous reporting nature of the FAERS database means that biases like under-reporting, incomplete reporting and false reporting might affect the conclusions. Third, it is challenging to determine the risk factors for ischaemic colitis among patients due to the lack of baseline indicators of gut health and information on concomitant medications. Nonetheless, the FAERS database persists as a critical resource for conducting drug surveillance analyses, offering valuable leads for upcoming prospective clinical studies.
Conclusions
This study identified 91 drugs associated with ischaemic colitis using the FAERS database, with the strongest signals observed for alosetron, tegaserod, osmoprep, naratriptan and kayexalate. Cases were more common in females and individuals aged ≥65 years, suggesting higher susceptibility in these groups. Two-thirds of the top 30 drugs lacked relevant warnings in their package inserts, indicating potential gaps in safety labelling. Many implicated drugs act on the gastrointestinal or nervous systems. These findings highlight the need for greater clinical vigilance and further research into the underlying mechanisms of the association between these drugs and ischaemic colitis.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Acknowledgments
This study was performed using open-source data provided by the FAERS database, and we thank all those who provided information for this database.
References
Footnotes
Contributors JA, TW, PX, CY, YF, QL and XD conceived the study. JA conducted the data analysis, wrote all sections of manuscript and edited the paper. TW, KW, YF and CY provided technical support for the analysis. KW, TW, CY, PX, QL, YF and XD were involved data acquisition. All authors reviewed and contributed to the final version of the manuscript. XD is the guarantor.
Funding The study was funded by the Shanxi Province '136' Revitalization Medical Project Construction Fund, the Key R&D Program of Shanxi Province (202102130501015) provided financial support, the Natural Science Foundation of Shanxi Province (202303021212329) and Research and Innovation Team Project for Scientific Breakthroughs at Shanxi Bethune Hospital (2024ZHANCHI06).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.