Article Text
Abstract
Introduction With the inspiring results of the PACIFIC trial in non-small-cell lung cancer (NSCLC), and the CAPIAN and IMpower133 trials in extensive-stage small-cell lung cancer (SCLC), immunotherapy has increasingly gained attention. Serplulimab, a PD-1 inhibitor, showed great antitumour activity in the ASTRUM-005 trial and has been recommended as first-line therapy in extensive-stage SCLC. Whether serplulimab following hypofractionation radiotherapy and chemotherapy could bring better outcomes in limited-stage SCLC remains to be answered.
Methods and analysis We designed a prospective multicentre single-arm phase II clinical trial to evaluate both the efficacy and safety of chemoradiotherapy and consolidation by serplulimab in limited-stage SCLC. Eligible patients will receive standard chemotherapy for four cycles and concurrent thoracic radiotherapy with a total dose of 45 Gy in 3 weeks and a 3 Gy dose per fraction. Prophylactic cranial irradiation is recommended for responding patients. Serplulimab will be delivered afterwards every 3 weeks for up to 1 year. Based on sample size estimation, 55 patients will be enrolled in total.
Ethics and dissemination Ethics approval was obtained from the Independent Ethics Committee of National Cancer Centre/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (22/236-3438).
Trial registration number NCT05443646.
- Respiratory tract tumours
- Immunology
- RADIOTHERAPY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This trial will be the first prospective study to assess hypofractionated radiotherapy combined with concurrent chemotherapy, followed by consolidation PD-1 inhibitor in limited-stage small-cell lung cancer.
This multicentre single-arm prospective study provides a defined treatment protocol and is helpful to obtain informed consent from participants, which is particularly important for promoting clinical research in China.
The efficacy evaluation will be completed separately by a blinded independent imaging centre and the investigators.
The limited sample size may restrict the ability to detect rare adverse events.
Introduction
Small-cell lung cancer (SCLC) originates from epithelial cells with neuroendocrine differentiation. It accounts for 15%–20% of the total number of lung cancers.1 SCLC is characterised by high malignancy, strong invasiveness, early metastasis and rapid disease progression, with an extremely poor prognosis. According to the Veterans Administration Lung Study Group (VALSG) stage, SCLC can be further divided into limited stage and extensive stage. Approximately 30%–40% patients are initially diagnosed with limited-stage SCLC (LS-SCLC).1 At present, surgery and chemotherapy with concurrent radiotherapy are standard treatment regimens for LS-SCLC. However, surgery is only applicable for a few patients with early SCLC, accounting for approximately 2%–5%. The combination of definitive thoracic radiotherapy and platinum-based chemotherapy followed by prophylactic cranial irradiation (PCI) in responsive patients has been the standard regimen in the past two decades. The median overall survival (OS) has been reported to be 25–30 months in recent years.2
The progress of SCLC research was slow in the 20th century until the encouraging results of the Impower133 study3 and CASPIAN study4 shed light on the treatment of extensive-stage SCLC (ES-SCLC). Thereafter, more PD-1/PD-L1 inhibitors were proven to be effective as first-line treatment for ES-SCLC.5–7 Among them, patients treated with serplulimab, the first PD-1 inhibitor showing efficacy as first-line systematic treatment according to the ASTRUM-005 trial, had the longest median OS time considering the absolute value (15.8 vs 11.1 months for placebo group; HR 0.62, 95% CI 0.50 to 0.76; descriptive p<0.001). Subgroup analysis of OS by race showed similar trends to improve survival in Asians and non-Asians.7
However, evidence of consolidation immunotherapy for LS-SCLC is limited. The STIMULI study, a phase II randomised controlled clinical study, failed to prove the superiority of consolidation immunotherapy consisting of nivolumab and ipilimumab.8 One possible reason for this may be the unexpectedly high rate of grade ≥3 adverse events (62% vs 25% in the experimental and observation arms, respectively). A randomised, placebo-controlled phase III trial, the ADRIATIC study, which investigated the efficiency of durvalumab following concurrent chemoradiotherapy in LS-SCLC, was ongoing when we designed this trial.9 It has recently represented a significant advancement in both median PFS (16.6 months vs 9.2 months, p=0.02) and OS (55.9 months vs 33.4 months, p=0.01).10 These results suggested the importance of the correct combination of immunotherapy and traditional chemoradiotherapy. With the inspiring results of serplulimab in ES-SCLC, we hypothesised that serplulimab might show promising efficacy in LS-SCLC with acceptable toxicity profiles.
Moreover, the optimal radiotherapy fractionation for LS-SCLC has been under debate. The hyperfractionation regimen (45/1.5 Gy twice daily/3 weeks) has been the standard regimen according to the Intergroup 0096 study since 1999.11 A new phase II trial suggested that a higher dose of 60 Gy in 40 fractions may further improve survival. However, this twice-daily mode significantly increased the occurrence of grade ≥3 acute oesophagitis compared with the once-daily mode (32% vs 16%, p<0.001 in the Intergroup 0096 trial). The increased load to patients, physicians and medical equipment of twice-daily radiotherapy is significantly limited in practice, and the once-daily schedule is more widely used.12 In the CONVERT trial and RTOG 0538 trial, the 66/70 Gy once-daily arm showed similar OS and toxicity to the 45 Gy twice-daily arm.13 14 Recently, several retrospective and prospective phase II studies showed that hypofractionated radiotherapy regimens with total doses ranging from 40 to 55 Gy were similarly effective as the 45 Gy twice-daily regimen, with similar incidences of grade≥3 acute oesophagitis and pneumonia.15–18 Both conventional and hypofractionated radiotherapy are recommended by the updated version of the National Comprehensive Cancer Network (NCCN) guidelines.
Additionally, a hypofractionated radiotherapy regimen may have a better synergistic effect with immunotherapy. Preclinical studies have suggested that a higher dose could remodel the tumour microenvironment by upregulating MHC-I expression, increasing CD8+T cell infiltration and upgrading the ratio of proinflammatory macrophages.19–21 However, myeloid-derived suppressor cells and regulatory T cells accumulate as well, together with induction of the DNA exonuclease Trex1, which attenuates the immunogenicity of cancer cells.22–24 Thus, hypofractionated radiation could potentially lead to a greater response to immunotherapy as a trade-off.
Survival has reached a plateau for patients with cytotoxic chemotherapy and radiotherapy. With the development of immunotherapy in recent years, it is acknowledged that the combination of radiotherapy and immunotherapy has a synergistic effect. It has been proven that radiotherapy induces immunogenic death in tumour cells, promotes the release and presentation of tumour antigens, activates cytotoxic T lymphocytes, returns tumour cells to the tumour environment, activates the immunogenicity of the whole body, reconstructs the tumour microenvironment and exerts a remote effect. In addition, immunotherapy normalises tumour blood vessels, relieves anoxia of the tumour microenvironment and makes tumours more sensitive to radiation.22
Synergy between radiotherapy and immunotherapy has been confirmed and applied in clinical practice in recent years. Previous preclinical studies suggested that a higher irradiation dose might trigger cell death more effectively, upregulate the expression of immunogenetic cell surface markers and better induce proinflammatory responses to enhance the clearance of tumour cells.19 25 26 Thus, hypofractionation might be a better complement to immunotherapy.
Based on previous evidence and urgent clinical needs, we designed this investigator-initiated multicentre phase II trial, the ASTRUM-LC01 study, to investigate the clinical efficacy and safety of serplulimab consolidation therapy after concurrent hypofractionated radiotherapy with chemotherapy in limited-stage SCLC.
Methods and analysis
Study design
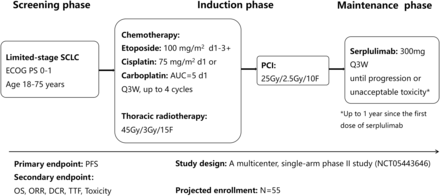
ASTRUM-LC01 will be carried out as a multicentre single-arm phase II clinical trial. Naïve LS-SCLC patients will receive concurrent chemoradiotherapy, PCI and subsequent serplulimab therapy. The flowchart of this study is presented in figure 1.
Flowchart of the ASTRUM-LC01 study. ECOG PS, Eastern Cooperative Oncology Group performance status; ORR, objective response rate; OS, overall survival; PCI, prophylactic cranial irradiation; PFS, progression-free survival; SCLC, small-cell lung cancer; TTF, time to treatment failure.
Research objectives
The primary objective of this trial was to evaluate improvements in PFS. PFS is defined as the time from the date of beginning of any antitumour therapy to the date of any documented disease progression or death due to any cause.
The secondary objectives include evaluating OS, objective response rate (ORR), time to treatment failure (TTF) and safety evaluation. OS is the period between the beginning of any antitumour therapy to any documented death due to any cause. ORR is the proportion of subjects achieving complete response (CR) and partial response (PR) as assessed by RECIST V.1.1. TTF means time to disease progression, death, withdrawal due to adverse events, patient refusal to continue the study or use of a new treatment from the very first date of treatment administration.
Participants
Detailed inclusion and exclusion criteria are presented in table 1. Patients with histologically or cytologically confirmed SCLC and staged at a limited stage were considered potential participants. Patients must be aged 18–75 years, with an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1, at least one evaluable lesion according to version 1.1 of the Response Evaluation Criteria in Solid Tumours, and no serious abnormalities in haematopoietic function or cardiac, hepatic or renal function or immunodeficiency. Patients who were suitable for surgery but refused were included. Detection of PD-L1 expression levels is highly recommended but not essential. All patients will be informed of the potential benefits and risks of this trial. Informed consent signed by every participant is requested.
Inclusion criteria and exclusion criteria
Treatments
All eligible patients will receive four cycles of chemotherapy after screening. The regimens are etoposide 100 mg/m2 intravenously on days 1–3 in combination with cisplatin 75 mg/m2 intravenously on day 1 (EP) or carboplatin AUC=5 every 3 weeks (EC). The selection of the regimen depends on the decision of the investigators. In cases of intolerance to chemotherapeutic agents, dose modifications are allowed twice according to the prescribing information for cisplatin/carboplatin and etoposide.
Thoracic radiation will be initiated no later than two cycles after chemotherapy.27 It is planned to target the primary tumour and involved mediastinal lymph node regions. For simulation, 4D-CT simulation positioning and enhanced CT positioning image scanning should be performed as much as possible. The scanning slice thickness was 0.3 cm. A total of 45 Gy in 15 fractions over 3 weeks will be delivered. PCI is routinely scheduled for patients without progression after chemoradiotherapy. 25 Gy in 10 fractions over 2 weeks to the brain will be prescribed. Image-guided intensity-modulated radiation therapy or volumetric modulated arc therapy techniques are needed. Treatment plans should meet organ dose constraints, as listed in table 2. Patients without disease progression will receive an intravenous infusion of 300 mg serplulimab every 3 weeks. Treatment with serplulimab should be continued until disease progression, intolerable toxicity, withdrawal of consent or a maximum of 1 year.
Organ at risk dose constraints
Assessment
Treatment response will be assessed every four cycles during serplulimab treatment with chest CT and brain MRI and ultrasonic examination when necessary. Contrast-enhanced CT of the neck, thorax and abdomen is preferred. Brain CT or PET is allowed if MRI is contraindicated. Each CT and MRI scan needs to be reviewed by radiologists and radiation oncologists. Subsequent assessments and follow-up will be performed every 6 weeks during the first 48 weeks of treatment and every 9 weeks after 48 weeks.
Patients will be interviewed face to face during their routine hospital visits and by telephone 1–2 weeks after delivery. Adverse events will be evaluated at each visit. Grades of side effects using CTCAE V.5.0 and their relationship with immunotherapy will be assessed by the research team members. Grade 2 pneumonia and grades 3–4 adverse effects will be managed by drug interruption and symptomatic treatment. Of note, dose reduction of serplulimab is not permitted.
Statistical considerations and analysis
The primary purpose of the phase II study was to evaluate the improvement in PFS for LS-SCLC patients. The study adopted the design of a superior test, and the sample size was calculated using historical data as a reference. The median PFS of LS-SCLC patients treated with standard CRT and PCI is approximately 15 months. The assumption of 80% power, a significance level of 10%, a HR of 0.65, an accrual period of 12 months and a minimum follow-up of 27 months resulted in a sample size of 50 individuals. Considering a maximum drop-out rate of 10%, a total of 55 patients will be recruited in ASTRUM-LC01. The sample size calculation was performed using the Power Analysis and Sample Size (PASS) software, version 15.
All collected data will enter the computer. Measurement data will be described as the median, mean value and SD. Enumeration data will be described as the number of cases and percentage. Kaplan‒Meier survival analysis and Cox regression will be applied for survival analysis. The log-rank method will be used for comparison between subgroups. A value of 0.05 was set as the statistically significant standard in this trial.
Monitoring
The study group will review data about treatment efficacy and safety. Trial monitoring is planned every half a year, and the monitoring committee will modify the protocol if necessary. Trial progress will be monitored by the study group, same as safety and protocol compliance.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research
Discussion
SCLC is a highly aggressive cancer with poor prognosis. Therefore, there is a great need to improve treatment efficacy, especially in the era of precision radiation and immunotherapy. Based on current evidence, immunotherapy has shown high efficacy as consolidation therapy in locally advanced NSCLC. The landscape of ES-SCLC treatment has also been significantly impacted by the increasing evidence of combining immunotherapy and chemotherapy in the first line. Whether consolidation immunotherapy would bring added benefits to traditional concurrent chemoradiotherapy in LS-SCLC has attracted much attention worldwide. This clinical trial was therefore designed to answer this question.
Serplulimab, a novel humanised monoclonal anti-PD-1 antibody, has emerged as a promising agent in this context. The ASTRUM-005 study demonstrated that serplulimab was the first PD-1 antibody to show positive results in the first-line treatment of ES-SCLC with advanced survival data, marking a milestone in the immunotherapy exploration process and the history of SCLC treatment.7 Meanwhile, ≥grade 3 adverse effects of serplulimab were similar to other drugs. Hence, we expect improved survival with acceptable toxicity in this trial, with great confidence.
The optimal duration of consolidation remains controversial. At the initiation of this trial in 2022, the only trial with results published, the STIMULI trial, adopted a similar design as the classic PACIFIC trial.8 Its failure was considered to be mainly due to treatment toxicity. The GEMSTONE-301 study and the recently published ADRIATIC study have opted for a longer duration of 2 years, and both significantly prolonged survival versus placebo.9 28 But the 2 year consolidation therapy in the GEMSTONE-301 trial did not demonstrate survival benefits beyond the PACIFIC pattern. Taking both the potential benefits of extended therapy and the costs into consideration, our study set the duration of serplulimab consolidation therapy to 1 year. But whether extended exposure to immunotherapy consolidation could potentially enhance its therapeutic effects will need further investigation.
Different from conventional or hyperfractionated RT in the ADRIATIC trial, hypofractionated thoracic radiation was scheduled during CRT in our study. This cost-effective regimen is permitted and recommended by NCCN guidelines. The synergistic effect of RT and immunotherapy might be dose-dependent, as mentioned before. Further research is necessary to define the precise parameters for combining RT with immunotherapy to achieve the best clinical outcomes.
There are some limitations to this trial. The single-arm design with small sample size may limit the generalisability of the findings. Also, since only Chinese patients will be enrolled, the applicability of the results to other regions with different healthcare systems and patient populations is limited.
In summary, this trial design comprehensively optimised treatment paradigm with the expectation of further improving the prognosis of LS-SCLC patients.
Ethics and dissemination
This study received ethical approval from the Independent Ethics Committee of National Cancer Centre/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (22/236-3438). This protocol has been accepted for poster presentation at the European Lung Cancer Congress (ELCC) 2024. Investigators will obtain written consent from patients willing to participate in the trial.
Ethics statements
Patient consent for publication
Acknowledgments
Serplulimab in this trial will be provided by Shanghai Henlius Biotech for free. This had no influence on the trial design, statistical analysis or publication.
References
Footnotes
YW and LD are joint first authors.
X @caolv2000
YW and LD contributed equally.
Contributors The study was designed by YW, LD, JC and NB. YW and JC developed the statistical analysis plan. YW and LD were major contributors in writing the manuscript. LD, JW, TZ, JC and XZ perform the research and monitor progress. JC, XZ and NB modified the manuscript. NB acted as guarantor.
Funding This study was supported by the Beijing Municipal Science and Technology Commission (no Z221100007422011).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.