Article Text
Abstract
Introduction The aetiology of sudden sensorineural hearing loss (SSNHL) is not certain in a significant number of cases. In 8%–31% of posterior fossa infarctions, acute hearing or vestibular loss precedes neurological symptoms. Also, several retrospective cohort analyses have indicated a higher chance of experiencing a stroke after SSNHL compared with the general population. This higher incidence of stroke suggests vascular involvement in the pathophysiology of SSNHL. The aim of this study is to evaluate the association of cardiovascular disease and idiopathic SSNHL (iSSNHL) by investigating the presence of cardiovascular risk factors and cerebral small vessel disease (CSVD), in patients with iSSNHL and compare this to controls.
Method and analysis In this multicentre cross-sectional study, the ROSALIE study, 205 patients aged 50 years or higher diagnosed with iSSNHL, and 205 controls who are either suspected of trigeminus neuralgia, hemifacial spasm, vestibular paroxysmia or have a cerebellopontine angle neoplasm will be included. The primary outcome is the difference in CSVD, measured by the degree of white matter hyperintensities according to the Fazekas scale and the presence of brain infarctions on MRI, between patients with iSSNHL and controls. The secondary outcome is the difference in prevalence of the cardiovascular risk factors: hypertension, hypercholesterolaemia, smoking status, body mass index and cardiovascular comorbidities; diabetes, stroke and myocardial infarction, between both cohorts.
Ethics and dissemination Ethics approval has been obtained by the institutional review boards of all participating hospitals. The Medical Research Involving Human Subjects Act does not apply to this study, as has been declared by the regional review board at Leiden University Hospital, registration number 22-3060.
Patients will receive the standard diagnostic protocol for iSSNHL in the Netherlands, which consists of pure tone audiometric assessment before and after treatment with corticosteroids and an MRI of the cerebellopontine angle displaying the entire cerebrum. The data will not be available publicly but might be shared on a reasonable request.
- Head & neck imaging
- OTOLARYNGOLOGY
- Audiology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
Multicentre study using hospital-derived data.
Radiological assessment by two expert neuroradiologists.
MRI sequences might slightly differ between participating centres.
Patients whose hearing has recovered by the time the MRI scan is scheduled will not be included in the study.
Introduction
Sudden sensorineural hearing loss (SSNHL) is an otological emergency, commonly defined as the onset of sensorineural hearing loss exceeding 30 dB in at least three contiguous audiometric frequencies, manifesting within a span of 72 hours.1–3
The annual incidence of SSNHL is approximately 5–20 cases per 100 000 individuals. In 32%–72% of the cases, the hearing loss recovers spontaneously.4 5 Unfortunately, in 30%–50% of cases hearing loss does not improve after treatment with high-dose corticosteroids, which is the generally accepted treatment modality in the Netherlands, based on the suspicion of an inflammatory aetiology.6
While the aetiological factor in most cases cannot be identified, one hypothesis under examination implicates vascular involvement in the pathophysiology of SSNHL. Given that the internal auditory artery is an end artery with little collateral blood supply, the cochlea is particularly susceptible to ischaemia.7 8 Research has focused on the potential link between idiopathic SSNHL (iSSNHL) and systemic cardiovascular disease, aiming to investigate the plausibility of vascular involvement in the pathophysiology of SSNHL. Multiple studies have reported higher prevalences of cardiovascular risk factors, such as smoking, alcohol abuse and hypercholesterolaemia in patients with SSNHL compared with controls.
Additionally, several cohort studies in Taiwan and Korea have reported increased incidence of stroke following iSSNHL.9–13 In a meta-analysis, Lammers et al. calculated the hazard risk of developing stroke after experiencing SSNHL to be 1.42.14 Kim and Lee reported that 8%–31% of patients experiencing posterior circulation cerebrovascular accidents exhibited preceding hearing or vestibular loss within a month.15 Sudden hearing loss might, therefore, be an indicator of stroke and warrant interventions to prevent cardiovascular events, known for their significant morbidity and mortality.
While the exact pathophysiology of cerebral small vessel disease (CSVD) is not clarified, it is widely believed to result from systemic cardiovascular disease, particularly hypertension.16 The presence of CSVD elevates the risk of stroke and other vascular neurodegenerative conditions, including vascular dementia.17–19 We hypothesise that patients with iSSNHL will exhibit a higher prevalence of CSVD than healthy individuals due to their increased cardiovascular comorbidity. CSVD can be visualised on MRI by the presence of white matter hyperintensities (WMHs), cerebral microbleeds, lacunes and silent brain infarctions.18
To investigate this hypothesis, we will conduct a multicentre cross-sectional analysis based on hospital-derived data. Our study aims to investigate the presence of WMH and brain infarctions on MRI in elderly patients with iSSNHL, comparing these findings with a carefully matched control cohort. Additionally, we will compare the prevalence of cardiovascular risk factors and comorbidity between both cohorts.
Method and analysis
Design
Patients will be included within a 2-year period starting from May 2023 at participating hospitals in the Netherlands. The participating centres are Gelre Hospital Apeldoorn and Zutphen, Leiden University Medical Centre, Groene Hart Hospital Gouda, Rijnstate Hospital Arnhem, Treant Emmen, Saint Franciscus Gasthuis Rotterdam, Medisch Spectrum Twente, Isala Zwolle and Rivierenland Tiel. A total of 410 patients will be included, 205 patients with iSSNHL and 205 subjects without iSSNHL in the control cohort. Patients will be recruited in the participating centres, but all data collection and subsequent analysis will be centralised and conducted at the Apeldoorn Dizziness Centre (ADC).
The ADC, located within the Gelre Hospital location Apeldoorn, serves as a multidisciplinary tertiary referral centre involving the neurology, otorhinolaryngology and clinical neurophysiology departments. The ADC specialises in the diagnostic and therapeutic workup of dizziness.
Inclusion
The study cohort will comprise patients diagnosed with iSSNHL and reside within the service areas of participating hospitals. A detailed description of the inclusion and exclusion criteria can be found in table 1.
Inclusion and exclusion criteria for participation in the ROSALIE study
The control cohort will consist of patients presenting at the ADC or Gelre Hospital with suspected diagnoses of trigeminal neuralgia, hemifacial spasm or vestibular paroxysmia, as well as patients with cerebellopontine neoplasms who have been referred to and are treated at the Leiden University Medical Centre.
Sample size calculation
A sample size calculation was conducted using nQuery software (Statsols, San Diego, CA, USA). Since the main outcome variable, the Fazekas score, is an ordinal variable, the sample size was calculated using a Mann–Whitney U rank-sum test with a two-sided significance level of 0.05. To perform this calculation, we used the proportions of patients falling into each Fazekas score category, as was previously observed in our retrospective case–control study comparing CSVD in patients with vestibular neuritis and a control cohort.20 The control population in this previous study aligns with the literature, where a Fazekas score of 2 is expected in individuals aged 60–70 years.21 Given our hypothesis of greater cardiovascular comorbidity in patients with SSNHL compared with the general population, we expect the median Fazekas score in the patients with SSNHL to be 3.
Figure 1 displays the output of the sample size calculation, demonstrating that the inclusion of 205 patients in each of the two groups (ie, SSNHL and controls) yields 80.72% power to reject the null hypothesis. This null hypothesis posits that the distribution of patients across the seven Fazekas categories in the SSNHL cohort and the control cohort is equal (ie, the latter is derived from our previous study20 and shown in the bottom part of figure 1).
The nQuery (Statsols, San Diego, CA, USA) sample size output shows that 205 patients in each group yield a power of at least 80%. The lower part of the figure displays the expected proportions of the seven ordinal categories (ie, Fazekas scores 0–6) for the SSNHL cohort and the control cohort that were used for the sample size calculation. p1 is the probability that an observation in controls(X) will be in a lower Fazekas score category than an observation in the SSNHL group (Y) when the alternative hypothesis is true. The null hypothesis being tested is that p1 = ½. SSNHL, sudden sensorineural hearing loss.
Study outcomes
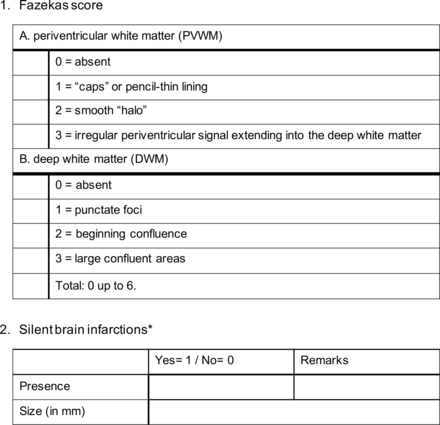
The primary objective of this study is to assess the difference in the prevalence of CSVD on MRI between patients diagnosed with iSSNHL and controls. This evaluation is based on two parameters: the extent of WMHs, quantified using the Fazekas score, and the presence of brain infarctions. The Fazekas score is the most frequently used diagnostic tool to assess the severity of WMHs in both periventricular and deep white matter regions.19 22 It is an ordinal scale ranging from 0 to 6, see figure 2. Brain infarctions are defined as lesions of the brain with a minimal diameter of 3 mm, displaying cerebrospinal fluid-like intensity on fluid attenuated inversion recovery (FLAIR) or T2 MRI sequences and clearly differentiable from leukoaraiosis and dilated Virchow–Robinson spaces.23
MRI assessment sheet. The Fazekas score is used to evaluate the severity of WMHs in both the periventricular and deep white matter. Brain infarctions will be scored in presence and size. WMHs, white matter hyperintensities. *SBI’s need to meet the following criteria: (1) Minimal size of 3mm; (2) Cerebrospinal fluid (CSF) appearance in all MRI sequences; (3) Can be differentiated from dilated Virchow-Robin spaces (dVRS).
The secondary outcome is the prevalence of the cardiovascular risk factors: age, gender, hypertension, hypercholesterolaemia, smoking status, body mass index (BMI) and cardiovascular comorbidities; diabetes, stroke and myocardial infarction. Hypertension is defined by meeting either of the following criteria: (1) having a medical history of physician diagnosed hypertension and/or (2) taking antihypertensive drugs. Hypercholesterolaemia is defined by meeting either of these criteria: (1) having a medical history of physician diagnosed hypercholesterolaemia and/or (2) the use of lipid-lowering medication. BMI is calculated by dividing the patients’ weight in kilogram by the square of their height in metres at the time of iSSNHL diagnosis. Smoking status is recorded as either former, current or non-smoker. Diabetes is defined by either having a medical history of physician diagnosed diabetes mellitus and/or the use of oral hypoglycaemic drugs or insulin.
Additionally, to account for potential confounding factors, binary and multinomial logistic regression analyses will be performed to compare the primary and secondary outcomes between both cohorts.
Treatment modalities
Corticosteroid therapy
The standard treatment for patients with iSSNHL is an oral corticosteroid regimen, consisting of 1 mg/kg/day of prednisolone with a maximum of 60 mg, administered for a period of 7–14 days. Subsequently, the dosage is gradually reduced to zero over the same timeframe.24 In case of a contraindication for oral corticosteroid use, intratympanic corticosteroid therapy is recommended. A 0.4 mL–0.8 mL injection of either dexamethasone (10 mg/mL) or methylprednisolone (30–40 mg/mL) is injected into the middle ear every 3–7 days, for a maximum of 4 sessions. If no significant improvement in hearing is observed following oral corticosteroid therapy, intratympanic corticosteroid injection can be considered for salvage therapy.
Study procedures
Subject identification and inclusion
Patients eligible for the study cohort will be recruited by their respective ear, nose and throat (ENT) surgeons at participating centres. Patients eligible for the control cohort will be recruited by neurologists or ENT surgeons at Gelre Hospital and ENT surgeons in Leiden University Medical Centre.
Data collection
Informed consent will be obtained during a telephone interview conducted several days after the patient receives the written patient information about the study. During this telephone interview, the patient will sign the informed consent form and return it to the research team at Gelre Hospitals through postal mail. The interview will also include the identification of the patient’s symptoms at the onset of iSSNHL, verification of their medical history and medication use.
Once the signed informed consent form is received by the coordinating investigator, relevant data from the participating hospitals will be sought. These data include the patient’s age, weight, height, medical history, current medication uses and their most recent MRI scan. For the SSNHL cohort, results from pure tone audiometric tests conducted before and after corticosteroid therapy, as well as results from video-head impulse testing and/or calorimetric tests, if performed, will be gathered. Additionally, details regarding the received treatment strategy will be collected.
MRI assessment
MRI scans will be included in the study if they were performed within 6 months prior to the onset of sudden deafness or within 6 months afterwards. In order to be adequately analysed, the MRI requires either a T2 or FLAIR sequence of the entire brain. While an MRI of the cerebellopontine angle or entire brain is a part of regular diagnostic workup, the scanning protocol might vary somewhat between participating centres. The scanning protocol, including types of sequences used, the TR (repetition time), the TE (echo time), voxel size, rotation and slice thickness for each patient, will be documented on finalisation of the study. Susceptibility-weighted imaging is commonly included in the scanning protocol of the MRIs included in our study. The main limitation thereof is included in the limitation section.
The MRI scans retrieved will be assessed by two neuroradiologists separately, each with multiple years of experience in MRI assessment of head and neck pathology. The radiologists will be blinded for clinical data. Figure 2 shows the scoring sheet used for MRI assessment. In case the degree of WMHs differs by two or more points on the Fazekas scale, the radiologist will review the MRI together until consensus is reached. Previous MRI assessments performed by both radiologists involved in this study demonstrated substantial interrater agreement in WMH assessment using the Fazekas score, with a kappa value of 0.74.20
Ethics and dissemination
The Medical Research Involving Human Subjects Act does not apply to this study, as has been declared by the regional review board at Leiden University Hospital, registration number 22-3060. Ethical approval for participation has been obtained by the institutional review boards of participating hospitals prior to the start of inclusion in these centres. The study received the following registration numbers according to participating centre: Leiden University Hospital: 22-3060; Gelre Hospitals: 2022-47; Groene Hart Hospital: LI-2023-05; Isala Hospital: 2023-0413; Medisch Spectrum Twente: KH23-23; Rijnstate Hospital: 2023–2207; Treant Emmen: 2023-16; St. Franciscus: 2022-47 and Rivierenland Tiel: 22-3060.
The final results of the study are planned to be published in an open access journal after completion of analyses. For information regarding the deposition of data, see the separate section.
Informed consent
Prior to seeking consent to enter the study, participants will receive an explanation of the study along with an information leaflet, followed by a minimum of 5 days for consideration. Participants have the right to decline participation without the need to provide a specific reason. If patients do not give consent in participating in the study, their contact information will thereafter be deleted from our records.
Data handling
Personal data, including medical history and diagnostic test results, will be sent to the investigating site via digitally protected email, after informed consent has been obtained.
On arrival at Gelre Hospital, the relevant information will be extracted from the received files. Subsequently, these data will be pseudonymised and stored in a research database, using Castor EDC (Castor EDC, Amsterdam, the Netherlands). The original files will be stored on a protected data drive at the ADC.
MRI scans obtained from participating hospitals will be shared electronically via the national Twiin platform for data exchange, developed by Vereniging van Zorgaanbieders voor Zorgcommunicatie, Den Haag, the Netherlands. The MRI scans will be pseudonymised and uploaded to a secured picture archiving and communication system (PACS) worklist at the radiology department of Gelre Hospital Apeldoorn. Following the assessment of the pseudonymised MRI scans, this PACS worklist will be deleted, while the MRI scans themselves have been stored on a protected data drive of the ADC.
The assembled document files, digital files and MRI scans will be saved for a period of 15 years on a protected data drive at the ADC. When this period has passed, all data will be deleted and all files will be destroyed.
Risks and benefits
Participants in this study will not be subjected to any additional study-related interventions beyond standard medical practices, such as MRI scans and audiometric testing, which are typically performed when SSNHL is suspected. No blood investigations or procedures beyond the established iSSNHL treatment guidelines in the Netherlands will be included in this study.
Implications for future research
The identification of vascular involvement in the onset of sudden deafness could have serious implications for the current treatment guideline of sudden hearing loss. Specifically, for a subset of patients that appears to have increased cardiovascular comorbidity, consideration of cardiovascular risk management, including anticoagulant administration, could be warranted. If the results of the cross-sectional study, as described in this article, provide evidence for vascular involvement in the pathophysiology of SSNHL, follow-up investigation of the included study population could be beneficial. This follow-up could imply a retrospective investigation of the incidence of stroke in the 5 years after inclusion in this study.
Data deposition
On reasonable request, the dataset and statistical code may be shared by contacting the primary author. The dataset will not be publicly accessible. To stimulate transparency and improve future research, this protocol will be published open access.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors All authors have contributed to the study design. FO, RBvL and TDB are responsible for the overall conduct of the study. TDB is the guarantor of this study. CC and FO will be responsible for the data collection. FO and TRS will be responsible for the statistical analysis. JJK and LMNP will be responsible for the MRI assessment. EFH and MJWL will be responsible for the inclusion of the control cohort.
Funding The data will be published according to the Strengthening the Reporting of Observational Studies in Epidemiology statement. The sponsor of this study (Gelre Hospital) will not interfere with public disclosure and publication of the research data.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.