Article Text
Abstract
Introduction The current diagnostic pathway for patients with a suspected inherited bleeding disorder is long, costly, resource intensive, emotionally draining for patients and often futile, as half of patients will remain without a diagnosis and be labelled ‘bleeding disorder of unknown cause’. Advances in understanding the genetic basis of the inherited bleeding disorders, coupled with both increasing infrastructure for genetic/genomic testing and decreasing costs, have increased the feasibility of introducing genomic testing into the clinical diagnostic pathway as a potential solution to improve the care of these patients. Yet, there remain evidence gaps on the optimal integration of genomic analysis into the diagnostic pathway.
Methods and analysis Using a multicentre randomised-controlled trial design, we will evaluate an early genomic testing strategy for the diagnosis of newly referred patients with a suspected inherited bleeding disorder. Eligible participants will be randomised to early genomic testing diagnostic pathway (intervention) or standard diagnostic pathway (control) and will be followed for a 12-month period. Patients in the control group who remain undiagnosed at study end will be offered identical early genomic testing to ensure equitable access to the intervention. The study will follow a parallel fixed design with waitlist control group and a 1:1 allocation ratio. The study will be conducted at three tertiary care centres in Ontario, Canada, with a target sample size of 212 participants. Clinical utility will be evaluated via the primary outcome of diagnostic yield, as well as the secondary outcome of time to diagnosis. Additional secondary outcomes will allow for assessment of patient impact via health-related quality of life and patient burden measures, as well as evaluation of economic impact through a cost-effectiveness analysis and budget impact analysis.
Ethics and dissemination This investigator-initiated study was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board through Clinical Trials Ontario (CTO-4909). Participant informed consent/assent is required. Findings will be disseminated through academic publications.
Trial registration number ClinicalTrials.gov, NCT06736158.
- Bleeding disorders & coagulopathies
- Genomic Medicine
- Clinical Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A major strength of this study lies in the randomised-controlled trial design, which will allow for a rigorous evaluation of the introduction of genomic testing into the diagnostic algorithm for inherited bleeding disorders.
The high-quality evidence generated from this study design carried out in a real-world setting of three tertiary care centres will provide invaluable insight into the optimal integration of this technology into hospitals/clinics and healthcare systems.
The diverse set of secondary outcome measures will allow for thorough assessment of the clinical, patient and economic impact.
The multidisciplinary research team approach brings together experts from many disciplines to provide a thorough evaluation (ie, haematology, genetics, health economics, statistics, nursing and laboratory testing).
A major limitation is the high likelihood of finding non-diagnostic variants of uncertain significance.
Introduction
Inherited bleeding disorders are characterised by a defect along the haemostatic response pathway that results in abnormal bleeding symptoms, ranging from mild nuisance bleeding to life-threatening haemorrhage. The challenges of diagnosing these rare disorders include issues of symptom dismissal, as well as difficulties inherent to the current specialised coagulation testing strategy.1 2 The diagnostic pathway begins with a detailed family history and clinical assessment, including obtaining a comprehensive bleeding history via a standardised Bleeding Assessment Tool (BAT).3–5 Use of a BAT results in a numeric bleeding score, classifiable as normal or abnormal with score magnitude reflecting bleeding severity. For patients suspected to have an inherited bleeding disorder (ie, abnormal bleeding history and/or family history of bleeding), the diagnostic pathway then continues to a sequential series of specialised coagulation tests.6
First-line testing will effectively diagnose approximately 30% of new referrals, skewed towards identification of von Willebrand disease and haemophilia A/B as opposed to the other rare inherited bleeding disorders.7 For the remaining 70% of referrals, subsequent rounds of coagulation and platelet function testing aim to identify platelet function disorders, rare factor deficiencies, and fibrinolytic disorders; however, tests are non-specific, have low sensitivity, low overall yield and do not evaluate the vascular component of the haemostatic response.6 All coagulation testing must be done in specialised coagulation laboratories, only found in large urban areas and not easily accessible by much of the population.8 Moreover, coagulation tests are variably affected by preanalytical factors (eg, transport time, maintenance of the cold chain, physiological stress, hormones) necessitating repetitive testing for validation of results.9 10 Other patients will not be able to proceed to further testing due to factors such as medication use or pregnancy which interferes with diagnostic accuracy and validity.10 For example, antidepressant selective serotonin reuptake inhibitors interfere with platelet function, thus patients taking these medications will be unable to complete the full diagnostic work-up, which includes platelet function testing, without a prolonged withdrawal of medically necessary therapy.
From the patient’s perspective, the burden of this diagnostic delay is significant as it includes multiple hospital visits, repeated venipunctures, days off work/school, travel and childcare costs, worry and uncertainty.1 It also includes years of living with untreated bleeding symptoms, including mucocutaneous bleeding (eg, epistaxis and oral cavity bleeding after dental procedures), prolonged bleeding after minor injuries or surgical procedures and gynaecological bleeding (eg, heavy menstrual bleeding, postpartum haemorrhage).11 The associated negative consequences include diminished health-related quality of life (HRQOL), work/school absenteeism, social isolation and excessive health-related costs.11–14 Furthermore, the absence of a definitive diagnosis limits the delivery of effective treatment. These negative effects are documented for patients with bleeding disorders across all severities, including bleeding disorder of unknown cause (BDUC).14 15
The current reported time from symptom onset to diagnosis of an inherited bleeding disorder ranges from 7 to 12 years for the 30% of patients who achieve a first-line diagnosis and even longer for patients who need second-line and third-line testing.1 Thus, the resultant diagnostic odyssey ends up being lengthy, costly, resource intensive, emotionally draining for patients and often futile, as up to half of patients will remain without a final diagnosis, despite a clear propensity to bleed.2 16 Approximately 50% of referrals end up with no definitive diagnosis and are classified as BDUC, defined as those with a positive bleeding score but in whom all current diagnostic test results are repeatedly normal.16 Managing bleeding complications in patients with BDUC is challenging as the specific bleeding aetiology is not known, and these patients have been shown to continue to experience major bleeding symptoms such as postpartum haemorrhage despite attempts at non-specific haemostatic interventions (eg, tranexamic acid).17
Advances in understanding the genetic basis of the inherited bleeding disorders, coupled with both increasing infrastructure for genetic/genomic testing and decreasing costs, have increased the feasibility of introducing genomic testing into the clinical diagnostic pathway as a remedy for these diagnostic challenges.18 Yet a major challenge for optimising integration is the wide variety of diagnostic yields reported, ranging from 10% to 94% depending on differences in study design (ie, prospective vs retrospective), inclusion criteria (ie, single condition studies vs all bleeding and coagulation disorders), the sequencing method used (panel vs whole exome sequences vs whole genome sequencing (WGS)), the number of genes assessed (ie, older studies have smaller numbers of genes assessed) and ways of reporting data (ie, only pathogenic variants vs both pathogenic and likely pathogenic variants). In a recent review paper, overall diagnostic yields were summarised as 95% for patients with clearly defined disorders on laboratory testing, between 50% and 70% for patients with less well-defined disorders on laboratory testing but well-characterised phenotypically and between 20% and 50% for those with poorly defined disorders.19
The observed variation in diagnostic yields raises questions of who should receive genetic analysis, which tests should be offered, and at what point in the diagnostic pathway they should be deployed.20 The integration of genetic/genomic testing into diagnostic pathways for inherited bleeding disorders varies across countries with many outstanding questions of optimisation. Real-world clinical studies are needed to produce data to determine the optimal integration of genomic analysis into clinical diagnostic pathways, including evaluations of cost-effectiveness and patient impact.
Aims and hypotheses
To evaluate an early genomic testing diagnostic pathway compared with usual care for patients with suspected inherited bleeding disorders along three domains.
Clinical utility: evaluation of the primary outcome diagnostic yield (the proportion of patients who achieve a diagnosis at 1 year), as well as time to diagnosis (time in days from initial appointment to diagnosis disclosure). We hypothesise our intervention group will have a higher diagnostic yield and a shorter time to diagnosis.
Economic impact: measured by cost-effectiveness analysis and a budget impact analysis. We hypothesise the relatively higher cost of genomic testing will be offset by savings related to fewer medical appointments/diagnostic tests.
Patient impact: evaluation of HRQOL and patient burden outcomes. We hypothesise that our intervention group will show a decreased patient burden and improved HRQOL related to less diagnostic uncertainty and improvement in symptom management.
Methods and analysis
Study design and setting
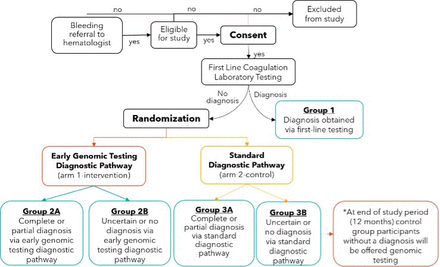
We will conduct a multicentre randomised controlled trial (RCT) where patients who do not achieve a first-line diagnosis with standard coagulation test screening will be randomised to early genomic testing diagnostic pathway (intervention) or standard diagnostic pathway (control) (figure 1). Patients in the control group who remain undiagnosed at study end (12 months) will then be offered identical early genomic testing to ensure equitable access to the intervention. The study will follow a parallel fixed design with waitlist control group and a 1:1 allocation ratio.
Trial schematic. First-line coagulation laboratory testing includes complete blood count, prothrombin, partial thromboplastin time, von Willebrand disease testing and coagulation factor levels.
Participants will be recruited from haematology clinics at three tertiary care centres in Ontario with established inherited bleeding disorder programmes: Kingston Health Sciences Centre (KHSC), St. Michael’s Hospital (SMH) and The Ottawa Hospital (TOH).
Eligibility criteria
Inclusion: (1) new patient referral for abnormal bleeding; (2) age of 12 years and older; (3a) haematology expert clinician determined abnormal bleeding history and family history of bleeding or (3b) no family history of bleeding but haematology expert clinician determined severe bleeding history.
Exclusion: (1) prior diagnosis of an inherited bleeding disorder; (2) acquired cause of bleeding (ie, medication known to cause bleeding, significant renal or hepatic disease).
Recruitment and data collection
At their initial appointment, eligible participants will meet with a research assistant at their initial appointment who will provide them with study information and complete the informed consent process. All consenting participants will complete baseline measures prior to randomisation via REDCap including: patient baseline questionnaire, Self-Administered Bleeding Assessment Tool and HRQOL measures (table 1). Participants will proceed to first-line testing as determined by their treating haematologist. Participants who receive a diagnosis with first-line testing will not be eligible for randomisation. All remaining patients will be randomised to intervention (early genomic testing) or control (usual care) (figure 1). A complete, concealed block randomisation schedule stratified by site21 was created by an independent researcher and uploaded to REDCap. Both participants and researchers will be unblinded to the randomisation result and be made aware of group allocation.
Summary of study measures with source and data collection time point
Participants will be followed for 12 months from the date of initial consultation and consent. The end of this 12-month period will be the second time point for data collection. Additional items including health resource utilisation data will be collected from participant medical records (table 1). Follow-up information will be collected directly from participants at the 12-month time point via REDCap including: the same HRQOL measures done at baseline and the P-GUIDE Questionnaire about their experience with genetic testing. Participants will be given a $25 gift card (canadian dollars) as a thank you for completing the 12-month follow-up questionnaires.
The study is expected to open in April 2025, with recruitment starting at that time. Participants can withdraw from the study at any time without having to provide a reason, without penalty. The study will only stop early if the sponsor decides or if the research ethics board withdraws permission for the study to continue.
Sample size
The sample size of 74 per arm was based on the following assumptions. It is anticipated that of the patients who proceed to randomisation (no first-line diagnosis), 30% of patients in the control group will receive a diagnosis within 1 year of enrolment.16 It is predicted that the addition of genomic testing in the intervention pathway will increase the proportion diagnosed within 1 year to 50%.22 Since the intervention group also receives the same non-genetic laboratory investigations as the control group, it is plausibly inconceivable that a lower diagnostic yield would be seen in the control group. Patients who drop out of the study or are lost to follow-up prior to receiving a diagnosis will be treated as not receiving a diagnosis. Therefore, we are using a one-sided type 1 error of 5%. Given these assumptions, in order to achieve 80% power to detect a 20% improvement in diagnostic yield, a sample size of 74 per group is needed.
The total aim is to recruit 212 patients over 1 year, 148 of whom we predict will not achieve a first-line diagnosis and will proceed to randomisation (n=74 for each study arm). Recruiting 212 participants per year is feasible given that approximately 300–350 eligible patients are seen annually at the three sites.
Intervention
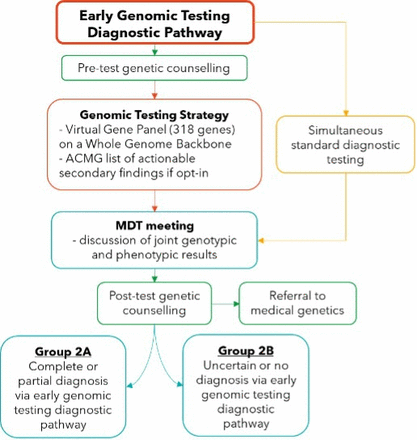
The early genomic testing diagnostic pathway (intervention) is outlined in figure 2.
Early genomic testing diagnostic pathway. ACMG, American College of Medical Genetics; MDT, multidisciplinary team; (L) BV, likely benign or benign variant (class 1–2 ACMG); (L) PV, likely pathogenic or pathogenic variant (class 4–5 ACMG); VUS, variant of uncertain significance (class 3 ACMG).
Optional secondary findings
All participants will have virtual pre-test genetic counselling with a certified genetic counsellor. After reviewing the benefits, limitations and potential outcomes of testing, participants will declare if they would like their results to be analysed for variants in the list of medically actionable secondary findings maintained by the American College of Medical Genetics and Genomics (ACMG). Secondary findings are purposely analysed but unrelated to primary testing indication. The current recommendation of the ACMG is that any time a person is receiving WGS, they should be offered the opportunity to have secondary findings also assessed.23 At the end of the genetic counselling session, participants who wish to opt in to secondary findings will complete a separate consent form through REDCap.
Genomic testing approach
Each sample will undergo WGS as the foundation for analysis. Not all of the data produced will be looked at, as analysis will focus only on identifying genetic variants possibly contributing to a bleeding disorder. A ‘virtual gene panel’ will be used comprising the most up-to-date list of genes known to be associated with bleeding, coagulation, platelet, connective and vascular disorders (online supplemental appendix A). The virtual panel comprises the International Society of Thrombosis and Haemostasis (ISTH) TIER-1 and TIER-2 gene list, as well as other genes identified in related scientific publications.24 25 The virtual gene panel will be updated annually, following publication of the updated ISTH gene lists.
Supplemental material
Any variants identified through examination of this panel will be evaluated by variant effect predictor analysis, in silico determination of effects on the phenotype, population frequency data and evidence from previously reported variants. The assignment of pathogenicity likelihood using the five classifications recommended by the ACMG26 will be determined using Varsome.27 If a (likely) pathogenic variant is identified, the significance of the variant will be further considered in terms of its pathobiological plausibility and alignment with the bleeding phenotype. Collectively, where a pathogenic variant is found in the virtual gene panel that also meets the additional requirements detailed above, this will be regarded as the cause of the inherited bleeding condition.
When examination of the virtual gene panel proves negative, additional genomic analysis may be done as needed. This may include: (1) review of additional variants: a review of other variants in the remaining genes; (2) evaluation of copy number variants through next-generation sequencing read depth; (3) family segregation studies: in cases where other affected family members are accessible and permission of the primary participant has been obtained, consistent segregation of the variant with the bleeding phenotype may also be evaluated. Family members will be consented separately and asked to provide a biological sample (eg, blood, saliva or cheek swab); (4) epigenetic changes: this analysis allows us to look for changes in the pattern of how genes are turned on and turned off, referred to as ‘epigenetic regulation’.
Sequencing and basic analysis will be done at The Centre for Applied Genomics at the Hospital for Sick Children in Toronto, Ontario. Subsequent analysis will be done at Queen’s University by team members affiliated with the National Inherited Bleeding Disorders Genotyping Lab. All patients who undergo genomic testing will be reviewed at a monthly multidisciplinary team meeting, comprising expert clinicians, medical and molecular geneticists, a genetic counsellor, and laboratory experts. Results of genomic testing will be reviewed along with results of any simultaneously conducted laboratory diagnostic testing to determine if a confirmed diagnosis can be made. A final genetic report will be issued detailing all genetic findings, primary as well as incidental findings that were opted in to by the participant. All participants with clinically significant variants or variants of uncertain significance will then have an individual appointment with a certified genetic counsellor for results disclosure and genetic counselling. Referral to medical genetics will also be done for clinical confirmation of research findings and arranging of any necessary clinical management. Disclosure of any bleeding disorder diagnosis and the recommended management will be provided by the treating clinician.
Control
Participants randomised to the control group will receive usual care as per the standard at each institution as determined by the treating haematologist. Final group classification (ie, complete/partial diagnosis vs uncertain/no diagnosis) will be done by the treating clinician and confirmed by a second independent expert clinician. After they have completed the study, patients in the control group will be offered identical early genomic testing, with the same pre-test and post-test counselling as described above.
Outcomes and analysis
Clinical utility (primary and secondary outcomes)
The primary outcome used to power the study is diagnostic yield, defined as the proportion of patients who achieve a complete or partial diagnosis at 1 year. Patients who drop out of the study or are lost to follow-up prior to receiving a diagnosis will be treated as not receiving a diagnosis. Therefore, there will be no missing data for the primary outcome. A secondary outcome will be the time to diagnosis, defined as the time in days from initial appointment with haematologist to patient disclosure of final diagnosis.
The primary outcome will be compared between groups using a one-sided Z-test comparing the proportions receiving diagnoses in each arm. The treatment effect will be reported as the absolute risk difference with a 95% CI. The secondary outcome of time to diagnosis will be analysed using time-to-event methods. Kaplan-Meier curves will be constructed and a proportional hazards model or suitable parametric model (eg, if the proportional hazard assumption is not reasonable) will be used to estimate the treatment effect.
Additionally, variables such as age, sex, symptoms and bleeding score will be explored as potential treatment effect modifiers (ie, subgroup effects) on the primary outcome by modelling the relevant interactions in logistic regression models.
Economic impact (secondary outcome)
An economic evaluation will be carried out alongside the RCT to evaluate the cost-effectiveness and budget impact analysis of the intervention. We estimate the intervention will be cost-effective due to an increased number of cases detected, and the higher cost of genomic testing offset due to reductions in the number of clinic visits and overall diagnostic tests with associated cost savings. Budget impact analysis conducted over 5 years will allow for estimation of the cost of implementing the new diagnostic strategy in Ontario.
Cost-effectiveness analysis
The cost-effectiveness analysis will be conducted from both the healthcare system (ie, the Ontario Ministry of Health) and the societal perspective (ie, all costs and benefits regardless of who pays and who benefits). Guidelines for economic evaluations of genetic and genomic testing state that the perspective should be defined by who is the decision-maker, which in the case of this proposal would be the Ontario Ministry of Health, who is the public payer for healthcare in this province.28 However, we also included the societal perspective as the same guidelines acknowledge that a value judgement can be made to consider including costs outside the healthcare sector, such as those borne by patients.28 As discussed above, coagulation testing can only be completed in specialised laboratories found in large urban areas and not easily accessible by much of the population.8 Patients are required to travel long distances to reach these specialised medical centres at their own expense, a process that must be repeated with each round of testing. Thus, in order to capture this eventuality, a societal perspective was added which includes all costs and benefits associated with the diagnostic pathway regardless of who pays and benefits.
The time frame for the cost-effectiveness analysis will be from April 2025 to April 2027, and participants will be followed for the 12-month time period. All costs and benefits will be reported in 2026 Canadian dollars, using inflation adjustment as per the Consumer Price Index for Canada.29 The primary outcome will be the number of cases detected, which is the most commonly used outcome in economic evaluations of genomic testing technology as a diagnostic tool.30–32 The main outcome of this analytic technique will be average cost per case detected of the intervention pathway vs the control pathway, expressed as the mean with 95% CI. Incremental cost per additional case detected will also be calculated and expressed similarly.
Outcome data will be captured through a case report form completed by research staff at each hospital at the end of the 12-month study period for each participant. Costs will be calculated prospectively based on resource utilisation related to diagnosis for each participant in the 12-month period. Costs associated with these resources will be accessed from the service provider in the case of genomic testing (TCAG), hospital decision support and financial services at KHSC and the Ontario Ministry of Health Lab Services Fees. Microcosting techniques may also be done as needed. Units of resource will be multiplied by price per unit. Indirect costs will be gathered from patient surveys where participants will answer questions about the time spent travelling to their appointment, whether they organised childcare and eldercare (and if yes, how much they paid), whether they took paid or unpaid time off work, etc. The total patient cost of attending an appointment will be calculated per patient and then multiplied by the number of appointments attended as part of their diagnostic pathway. As this is a diagnostic study, we will not be looking at medical care costs outside of those used for the purpose of diagnosis (ie, clinic appointments and all testing including lab and genetic)
Uncertainty will be evaluated via one-way sensitivity analyses on key parameters including cost of genomic testing, number of cases detected and savings due to averted clinic appointments, with additional subgroup analyses also conducted on key parameters.
Budget impact analysis
We will conduct a budget impact analysis from the Ontario Healthcare System perspective over 5 years similar to genomic testing programmes for other conditions that have been evaluated by Ontario Health Technology Assessment Committee.32 Standard budget impact analysis techniques will be used33 to predict the future economic impact of genomic testing over 5 years from 2025 to 2030.
In this model-based analysis, the incremental cost of testing for both the control and intervention arm will be determined, which will allow for detailed analysis on the economic impact of inserting genomic testing at different time points along the diagnostic algorithm. This will account for the fact that by the second time point (1 year post initial consultation), some patients in the control arm will not have had sufficient time to complete the full diagnostic work-up and thus the full cost of their diagnostic journey will not be captured. This will provide further evidence of the feasibility and optimal timing of genomic testing. The same methods for prospective cost estimation delineated above will be employed, excluding patient incurred costs, with similar sensitivity analyses conducted on key parameters.
Patient impact (secondary outcomes)
The impact of the intervention on patients will be evaluated via generic HRQOL measures (ie, Patient-Reported Outcomes Measurement Information System Questionnaire (PROMIS)), symptom-specific HRQOL (ie, Menstrual Bleeding Questionnaire (MBQ)/adolescent Menstrual Bleeding Questionnaire (aMBQ)) and a patient-reported genetic testing utility measure (ie, Patient-Reported Genetic testing Utility InDEx (P-GUIDE)). Additional patient burden indicators related to the diagnostic journey include total number of appointments for diagnosis, total number of blood draws, transfusion information, travel items (distance, mode, associated costs) and productivity loss questions (eg, time spent away from work, wages lost, child/elder care costs).
The varied nature of these outcomes necessitates a variety of analytic methods for between-arm comparisons. Models for count data (eg, Poisson, negative binomial, etc) will be used when deemed appropriate (ie, number of appointments, number of blood draws). Rate ratios and 95% CIs will express the intervention effect. Other analyses will involve simple comparisons of means (eg, t-test or non-parametric equivalent) as needed. The intervention effect will be expressed as mean difference with 95% CI (or another appropriate difference if t-test assumptions are problematic). HRQOL assessment at 1 year will be analysed by linear regression, adjusted for the baseline value and the treatment effect will be the adjusted mean difference with 95% CI.
Data management and monitoring
All data collection activities will be coordinated from KHSC. Participant recruitment, consent and usual care clinical visits will take place at participating hospitals (KHSC, SMH and TOH). Email contact information will be submitted by the participant into REDCap for 12-month follow-up data collection. Data from medical charts will be abstracted on site and entered into REDCap by the local research team. The study REDCap database is hosted by the Centre for Advanced Computing at Queen’s University. The final trial dataset will be placed in an open-access, publicly accessible repository.
Long-term storage of data from genome-wide sequencing will be stored in the Care4Rare Canada Genomics4RD Research database.34 The genome-wide sequencing data stored in Genomics4RD will be coded so that no directly identifying information of study participants will be associated with these dataset records.
There will be no interim analyses and no data safety monitoring board as we are conducting a diagnostic clinical trial only, not involving high risks nor diseases with high mortality or morbidity.
Patient and public involvement
MCo is a patient representative and involved in the design of the study protocol.
Ethics and dissemination
This protocol was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (HSREB) through Clinical Trials Ontario (CTO-4909), the provincial platform responsible for approving trials involving two or more academic or healthcare institutions. Informed consent will take place at the initial haematology appointment by trained research staff with the full consent form reviewed, questions answered and the participant given a copy of the consent form for their records (online supplemental appendix B). Paediatric participants under the age of 18 who do not have the capacity to consent will provide assent as per ethics regulations, with their parent/guardian providing consent (online supplemental appendix C). For participants who would like the optional analysis of the ACMG list of actionable secondary findings, after pretesting counselling with a certified genetic counsellor, a secondary consent/assent form will be reviewed virtually with the participant and informed consent obtained virtually through REDCap (online supplemental appendices D and E). The consent form will ask permission to recontact participants for future research studies. All protocol modifications will be communicated to relevant parties as per Queen’s HSREB guidelines.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Guidelines for incorporating genomic testing into diagnostic algorithms for patients with suspected inherited bleeding disorders have been published and will be followed with clear recommendations surrounding informed consent, management of incidental findings and clinical interpretation of variants.35 36 Although full consent for the study will be initially obtained, our study design, which delays the opt-in choice until after the pretest counselling with the certified genetic counsellor, serves to ensure full understanding of implications prior to declaring opt-in to secondary findings. Participants will also have post-test counselling and referral to a medical geneticist. For paediatric patients under the age of 18 years, families will provide consent with a separate assent collected from the patient.
The results of this study will be communicated via traditional methods including conference presentations, published abstracts and publication of peer-reviewed manuscripts. Additional knowledge translation activities will include the presentation of results to key stakeholders including the Ontario Ministry of Health, the Association of Hemophilia Clinic Directors in Canada and the Canadian Association of Nurses in Hemophilia Care, for the incorporation of results into national diagnostic guidelines for the diagnosis of inherited bleeding disorders.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors PDJ conceived the study and is the guarantor. MCh, MB and PDJ designed the protocol. MB, JG, AJ, KT, AG, MCo, JL, MS, DL, RK, AP, DG, AM, JC, and RD informed the design of the protocol. MCh drafted the manuscript. All other authors reviewed, revised and approval the final manuscript.
Funding This work is supported by a Canadian Institute of Health Research Team Grant (RDP-193724). This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data or decision to submit results.
Competing interests PDJ receives research funding from Bayer and consultancy fees from Star/Vega Therapeutics, Band/Guardian Therapeutics, Roche and BioMarin. JL has received honoraria from CSL Behring, Novo Nordisk and Bayer. RK has received honoraria/support from Bayer, Pfizer, Novo Nordisk, Sanofi, Takeda and Roche. MS receives research funding from Pfizer and Octapharma, and honoraria from Octapharma, Sobi, Werfen and Roche. DL receives research funding from BioMarin, CSL-Behring and Octapharma and consulting fees from BioMarin, CSL-Behring, Novo Nordisk, Pfizer and Sanofi. JC receives research funding from Canadian Blood Services and Octapharma. All other authors have no disclosures.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review Not commissioned, peer reviewed for funding approval through Canadian Institute of Health Research and ethical approval through Queen’s University HSREB and CTO prior to journal submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.