Article Text
Abstract
Introduction As a mind–body exercise, Yijinjing has shown benefits in enhancing the effects of manual therapy for the treatment of pain, disability and soft tissue status associated with non-specific chronic neck pain (NCNP). The efficacy of Yijinjing as an independent exercise regimen for the treatment of NCNP has not been established. This study is designed to assess the efficacy of Yijinjing in patients with NCNP, compared with cervical function training (CFT).
Methods and analysis A total of 132 consenting NCNP participants will be randomly assigned in a 1:1 ratio to either the Yijinjing group or the CFT group (three times a week for 8 weeks). Both groups will undergo an 8-week intervention phase. Outcome variables will be assessed at baseline and at 4-week, 8-week and 12-week follow-up. The primary outcome measure is the change in visual analogue scale scores at week 8. Secondary outcomes include neck disability index, cervical range of motion and soft tissue status parameters.
Ethics and dissemination This study has been approved by an independent ethics committee and will be carried out according to the principles of the Declaration of Helsinki, local laws and regulations. The results of this study will be disseminated through presentation at scientific conferences and publication in peer-reviewed journals.
Trial registration number ITMCTR2024000323.
- Randomized Controlled Trial
- Physical Therapy Modalities
- SPORTS MEDICINE
- Exercise
- Musculoskeletal disorders
- Chronic Pain
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Randomized Controlled Trial
- Physical Therapy Modalities
- SPORTS MEDICINE
- Exercise
- Musculoskeletal disorders
- Chronic Pain
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study is conducted in three tertiary hospitals in Shanghai to minimise bias from different hospital settings.
The study followed the principle of separation to regulate the behaviour of researchers and participants, reducing potential bias.
All cases were sourced from the Shanghai region, which may limit the generalisability of the study results.
Introduction
Background and rationale
Non-specific chronic neck pain (NCNP) refers to neck pain lacking clear diagnostic evidence, excluding specific conditions, such as radiculopathy, myelopathy or whiplash injury, and persists for more than 3 months.1 The Bone and Joint Decade 2000–2010 Task Force found that the global point prevalence of neck pain ranges from 15.4% to 45.3%.2 A longitudinal cohort study in the USA revealed that approximately 40% of these cases are attributed to NCNP.3 4 In the 25–49-year-old age group, neck pain ranks 19th among 369 diseases in terms of disability-adjusted life years, significantly impacting patients’ quality of life.2 5 Typical features of NCNP include pain, altered soft tissue status and restricted cervical range of motion (CROM).6 7 Exercise therapy is a clinically recommended strategy for treating NCNP.5 8 Clinicians often use cervical function training (CFT) programmes to alleviate symptoms. CFT programmes typically include a combination of neuromuscular training, strength and endurance exercise and stretching, with proven efficacy.9 10 Stability training, which integrates neuromuscular control, strength and endurance training, is widely used in clinical practice.10 Increasing research confirms the effectiveness of mind–body exercises, such as Tai Chi and yoga, in alleviating neck pain.11 12 In contrast, Yijinjing is a mind–body exercise that targets muscle strength, endurance and coordination.13 Yijinjing requires no specialised equipment or space.14 Cheng et al’s study demonstrated that the combined use of Tuina and Yijinjing resulted in superior improvements in visual analogue scale (VAS) pain reduction, neck disability index (NDI), tissue hardness and active range of motion compared with the Tuina-only group.14 The study also confirmed the safety of Yijinjing, with no adverse events reported among the 51 patients who received the Yijinjing intervention. However, comparative studies on the efficacy of traditional Chinese mind–body exercises versus CFT for the treatment of NCNP remain limited.12 15 16 The objective of this study is to evaluate the potential efficacy of mind–body exercises, such as Yijinjing, in treating NCNP and to evaluate whether incorporating such exercises into clinical practice could inform future clinical guidelines or recommendations for NCNP management.
Methods and analyses
Study design
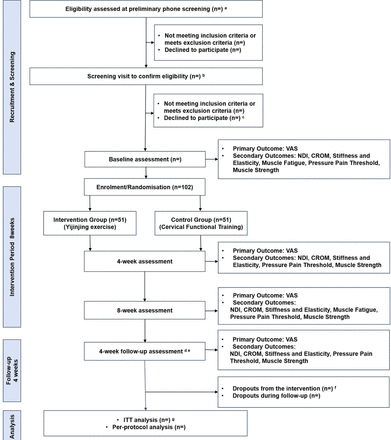
The proposed study is an assessor blinding, parallel-group, stratified block randomised RCT. It will be conducted at three tertiary hospitals in Shanghai, China: Shanghai Municipal Hospital of Traditional Chinese Medicine (TCM), Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine (SHUTCM) and Yueyang Hospital of Integrated TCM and Western Medicine affiliated to SHUTCM. These hospitals are all affiliated with SHUTCM and are well equipped to provide comprehensive support for the study. Eligible patients will be fully informed of the potential benefits, risks and responsibilities through an informational sheet. Participants who provide consent will be randomly assigned to either the Yijinjing group or the CFT group. Both groups will undergo training three times a week for 8 weeks, with a 4-week follow-up, based on the prior research conducted by our team and relevant meta-analyses on exercise training.14 17 During the intervention, all participants will be assessed for VAS scores, NDI, CROM and soft tissue status, and adverse events will be recorded. 4 weeks after the intervention ends, participants will be followed up and the relevant outcomes will be assessed (figure 1 and table 1 provide detailed trial procedures).
Study procedure flow diagram. CROM, cervical range of motion; ITT, intention-to-treat analysis; NCNP, non-specific chronic neck pain; NDI, neck disability index; sEMG, surface electromyography; VAS, visual analogue scale. a. The preliminary phone screening will assess the participant’s willingness to participate in the study, along with key details, such as age, the presence of NCNP, duration of symptoms and any medical history that may exclude the participant from being eligible for exercise therapy. This phase will also assess their engagement in regular exercise and other relevant factors. b. During the screening visit, the participant’s willingness to participate will be reconfirmed, along with a detailed review of their medical history, including prior illnesses, treatments, laboratory tests and imaging results (if applicable). Additionally, a comprehensive physical examination will be conducted to ensure the participant’s suitability for inclusion in the study and exercise therapy. c. Reasons for declining participation will be documented, if applicable. d. Participants were instructed to refrain from any neck-related exercises during this period to minimise potential biases in follow-up outcomes. e. Follow-up will primarily be in person, with virtual follow-up via voice calls or online surveys for participants unable or unwilling to attend in person. f. Attempt will be made to follow-up with dropouts. Follow-up will be prioritised in person, with virtual follow-up (via voice calls or online surveys) for participants unwilling to attend in-person assessments. g. Multiple imputation techniques will be employed to replace randomly missing data.
Schedule of enrolment, interventions and assessments
Patient and public involvement
Before designing this trial, the principal investigator consulted with clinicians and considered feedback from patients with NCNP. The frequency and duration of the treatment were determined based on the standard protocols for exercise interventions, expected patient compliance and the feasibility of the study. This approach ensures both scientific validity and practical applicability.
Eligibility criteria
Inclusion criteria
Eligible participants must meet all the following inclusion criteria: (1) neck pain persisting for more than 3 months, with intermittent pain occurring at least once a week; (2) aged between 20 and 60 years; (3) irrespective of gender; (4) exhibiting characteristics of non-specific neck pain, with no specific signs of neurological damage or whiplash injury, as defined by the British Medical Journal guidelines1 3; (5) an average VAS pain score between 3 and 8 during the past week to exclude cases with either too mild or too severe symptoms and (6) agreement to participate in the study and signing of the informed consent form.
Exclusion criteria
Participants will be excluded from this trial if they meet any of the following conditions: (1) other cervical spine diseases or medical history, such as cervical fractures, postcervical surgery status, fibromyalgia or spasmodic torticollis; (2) not meeting the diagnostic criteria for non-specific neck pain, such as the presence of specific signs of radiculopathy, myelopathy or whiplash injury; (3) diseases that make them unsuitable for exercise training, such as shoulder injury, arm injury, severe osteoporosis, severe liver or kidney dysfunction, severe cardiovascular disease, severe diabetes or psychiatric disorders; (4) diseases that can cause neck pain and affect cervical function, thereby impacting assessments, such as cancer, migraines or rheumatic diseases; (5) pregnant or breastfeeding women; (6) participation in other studies within the past 3 months; (7) consistent exercise training within the past 3 months, defined according to the WHO guidelines as more than 150 min of exercise per week18 and (8) inability to read, write or speak Chinese.
Participants recruitment
To comprehensively recruit patients, each hospital will provide independent offline posters, flyers and online ads. Additionally, doctors from each hospital will regularly visit communities to conduct educational sessions on NCNP and distribute recruitment flyers specific to their hospital. These efforts ensure that non-tech users and individuals with limited hospital access can receive trial information. Furthermore, potential participants may obtain hospital poster or flyer contact information through friends, allowing broader accessibility. However, patients with mobility restrictions are not eligible for recruitment, as they do not meet the inclusion criteria for the exercise-based intervention. Participants can contact the trial coordinator via phone or QR code, which are provided on offline posters, flyers and online ads. Based on the contact information of the corresponding hospital in the recruitment advertisement, the trial contact person will discuss the trial in detail with potential participants and conduct an initial telephone screening to determine eligibility. If the initial screening indicates eligibility, the trial contact person will arrange a screening visit at the respective hospital. Participants who meet the inclusion criteria and have no exclusion conditions will discuss further with the trial contact person regarding their willingness to join the study. Participants who are willing to participate in the study will sign the informed consent form, the details of which are provided in Data Supplement 3. Those who agree and sign the informed consent form will undergo a comprehensive baseline assessment.
Sample size calculation
Based on the results of previous studies conducted by other researchers,10 19 the expected effect size for the primary outcome measure, VAS pain score, was determined. We hypothesise that the mean improvement in the Yijinjing group will be 29 (SD=13.5), while the mean improvement in the CFT group will be 16 (SD=22). Using an independent samples t-test with a significance level of 0.05 and a power of 90%, we initially calculated that 86 patients with neck pain would be required. Considering a 15% dropout rate, which was selected based on the dropout rates observed in other non-pharmacological therapies and the actual dropout rates in the research team’s previous studies, as well as the requirements for stratified block randomisation with variable block sizes set at 4 or 6, we determined that a total of 102 patients with neck pain would be needed, resulting in 51 patients per group.20
Randomisation
Recruited participants will begin intervention within 2 weeks of baseline assessment. We will use a stratified block randomisation method, with a block size predetermined as 4 or 6, and stratification based on the intervention and assessment hospital of the participants to ensure the balance between the Yijinjing group and the CFT group. Participants will be randomly assigned within each hospital separately, with different randomisation schemes applied at each site to minimise the potential for site-specific biases. The randomisation sequence will be generated by an independent statistician using statistical software (R 4.3.3).
Allocation concealment
To ensure allocation concealment, clinical coordinators will use sealed opaque envelopes (SOEs) to contain the randomisation sequence. Independent SOEs will be distributed to the trial contact person at each participating hospital. The unique code inside the envelope will be generated by a statistician who is not involved in the study, ensuring impartiality and preventing any bias in group allocation. The envelope will be labelled on the outside with the participant’s name, hospital, contact information and a sequential number to ensure proper distribution. Inside the envelope, the group assignment will be indicated by the unique code. Before the study’s initiation, the clinical coordinator will maintain a group allocation table linking the unique codes inside the envelopes to the corresponding group assignments. After participants fill out their personal information on the outside of the envelope, the trial contact person will provide the clinical coordinator with the participants’ information and unique code. The clinical coordinator will use the unique code to determine the participants’ group assignment and arrange for the treatment staff of the corresponding intervention to contact the participant assigned to that intervention.
Blinding
Due to the limitations of the intervention in this trial, treatment staff will be aware of participants’ group assignments. We will implement various measures to ensure that all research staff, coming from different departments, are blinded to each other’s involvement in the study at every stage. To ensure blinding, all research staff will undergo multiple training sessions on study protocol implementation before the trial begins, and strict adherence to the principle of departmental separation will be required. Before the start of the study, all staff will undergo training to avoid unnecessary information exchange between departments and to follow the necessary procedures for participant management. Assessments and treatments will take place in different rooms to ensure that evaluators remain blinded to the participants’ intervention groups. Participants will only be informed about the specific steps of their own group’s intervention and will remain unaware of the other group’s treatment procedures. Treatment staff must not disclose intervention details to other researchers and will instruct participants not to reveal or discuss their intervention with others. In addition, the data monitoring committee (DMC) will conduct quarterly reviews to assess the effectiveness of these separation measures and ensure their proper implementation throughout the study. The principal investigator, trial contact person, evaluators, data entry staff and statisticians will remain blinded throughout the study to ensure objectivity and data integrity.
If a participant voluntarily withdraws from the trial for personal reasons, the DMC will confirm and record the participant’s group assignment. No other unblinding will occur during the trial except under these circumstances.
Withdrawal
If participants experience changes in their condition during the study, they must report this to the treatment staff at their respective hospital, where it will be initially recorded. Treatment staff will report any adverse events to the DMC, and the reporting will follow the Guidelines for Adverse Event Identification and Reporting, as detailed in . The decision on whether the participant should be withdrawn from the clinical trial will be based on the following criteria. (1) Exacerbation of Primary Symptoms: if the participant’s primary symptoms (eg, NCNP) significantly worsen, severely impairing daily functioning or safety. (2) Need for Emergency Medical Intervention: if the participant requires emergency medical intervention (eg, hospitalisation, surgery or emergency care) due to the study intervention or the underlying condition. (3) Unanticipated Serious Adverse Events: any unexpected, severe adverse events related to the study, such as trauma, allergic reactions and severe pain, that pose a threat to the participant’s health and safety. (4) Risk to Participant’s Health: if continued participation in the trial poses a significant risk to the participant’s physical or psychological well-being, as determined by both the treating physician and the DMC. In such cases, if the DMC assesses that the participant meets any of the above criteria, it will decide on the participant’s withdrawal from the clinical trial. Participants also have the right to withdraw consent and exit the trial at any time. On withdrawal from the study, participants will be asked to provide a reason for their decision (although not required).
Intervention
The Yijinjing and CFT will be conducted independently at three hospitals. Each hospital’s training session will follow the same protocol and be led by trained treatment staff to ensure consistency. Each training session for both exercise programmes will last 30 min, a duration chosen based on the feasibility of patient participation and balanced with the time allotted for both interventions. This training time was established by referencing previous studies on optimal exercise duration.21 Multiple studies have investigated the effects of various exercise protocols on NCNP. According to the systematic review by Rasmussen, CFT interventions typically last 4–10 weeks, while mind–body exercises range from 4 to 12 weeks, with a training frequency of 2–4 sessions per week, each lasting 20–60 min. Among these, a regimen of three 30-min sessions per week for 8 weeks is commonly used in both CFT and mind–body exercise studies.22 Additionally, randomised controlled trials by Gaban et al implemented this intervention frequency for CFT and Yijinjing, respectively, yielding positive results.23 Standardising the duration and frequency of both exercise modalities facilitates a balanced comparison of their effects. The treating physician will work with participants to ensure that two training sessions are conducted at the hospital and one session is completed at home each week, with a minimum interval of 24 hours between sessions. The tools required for CFT will be distributed to participants before the intervention begins. Participants will be instructed to inspect the resistance bands and exercise balls for any defects before use. The procedures for inspecting and using the equipment will be standardised to ensure consistent usage across all participants. These instructions will be provided on standardised paper. The entire intervention will last for 8 weeks. Participants are not advised to receive other treatments during the intervention period; however, this is not strictly prohibited. Any additional treatments, including their type and dosage, will be documented. Participants will receive identical health education before the intervention to prevent poor cervical posture, which includes avoiding vigorous acceleration–deceleration activities, considering physiological curvature when using pillows to provide adequate support for the cervical spine, and avoiding prolonged maintenance of forward head posture, head flexion, tilting or slouching.
Yijinjing group
The Yijinjing intervention is based on textbooks from TCM institutions and includes five specific movements, with reference to the previous research conducted by our team: Wei Tuo Xian Chu Third Form, Zhai Xing Huan Dou Form, Jiu Gui Ba Ma Dao Form, Da Gong Form and Diao Wei Form.13 14 24 These five movements collectively incorporate multidirectional stretching and stability exercises. Movements were selected based on their general rehabilitative qualities. Online supplemental figure S1 and online supplemental 2 provide detailed descriptions of the implementation steps for these Yijinjing movements. The image consent form for online supplemental figure S1 is provided by the image provider in Data Supplement 5.
Supplemental material
Supplemental material
CFT group
The CFT is based on the corrective exercise guidelines from the National Academy of Sports Medicine (NASM) and relevant literature and consists of four exercises focusing on strength, stretching, neck stability and core stability.10 25 Online supplemental figure S2 and online supplemental 2 provide detailed descriptions of the CFT components. The image consent form for online supplemental figure S2 is provided by the image provider in Data Supplement 5.
Supplemental material
Outcome measures
Primary outcome
This RCT aims to investigate whether Yijinjing can more effectively improve symptoms in patients with NCNP compared with conventional cervical spine functional training. The primary outcome measure will be the change in pain assessed using the VAS at week 8. The VAS is a 100-mm horizontal line with endpoints representing ‘no pain’ (0) and ‘worst pain’ (100), allowing participants to self-report their pain intensity.26 27 This scale is simple and effective, and is one of the most commonly used tools for pain assessment in clinical practice, with good sensitivity and specificity.28 Based on the previous studies, the minimal clinically important difference for NCNP is defined as an 8 mm change on the VAS.29
Secondary outcomes
Neck disability index
NCNP can affect various aspects of daily functioning. We will use the NDI to assess the extent of functional impairment caused by neck pain. The NDI covers ten different domains, including pain intensity, personal care, ability to lift weights, reading, frequency and severity of headaches, cognitive concentration, work function, sleep quality, driving ability and participation in recreational activities. The NDI has good reliability and validity, making it an important tool for measuring the degree of cervical spine disability.30 To minimise response bias, participants will be instructed to complete the NDI independently. The NDI score will be used to assess the level of neck pain disability, but not to classify its severity.
Cervical range of motion
Patients with NCNP may experience varying degrees of restricted movement. To evaluate the effectiveness of Yijinjing on CROM, we will use a goniometer (Theratools, USA) to measure the range of active cervical flexion, extension, lateral flexion and rotation performed by the participants.31 We will use the same evaluator for all measurements. The evaluator will undergo standardised training to ensure that the measurement procedures are standardised. Before each test, participants will be instructed to align their ears in the same horizontal plane, ensuring that the earlobes are vertically in line with the acromion, to maintain a neutral head position.
Soft tissue status
Measurement of soft tissue status will be conducted using different portable soft tissue assessment devices. The selection of measurement points follows the SENIAM standards established by the European Biomedical Health Research Programme (http://seniam.org/) and relevant literature.32 33 We will measure six points bilaterally on the muscle bellies of the splenius capitis, upper trapezius and middle trapezius muscles: 2 cm lateral to the C5/6 spinous process (splenius capitis), the midpoint between the C7 spinous process and acromion (upper trapezius) and the midpoint between T3 and the medial border of the scapula (middle trapezius).
Stiffness and elasticity
We will use the OE220 outcome evaluation system (ITO, Japan), which comprises a tissue hardness metre, to assess the numerical values of stiffness. Meanwhile, we will use the MyotonPRO Digital Palpation Device (Myoton AS, Estonia). This device sends measurement pulses to the surface of the target area and automatically records five key parameters to assess soft tissue status from multiple dimensions. Two parameters associated with stiffness include natural oscillation frequency and dynamic stiffness. Natural Oscillation Frequency: this parameter measures the inherent frequency of free oscillation of the soft tissue in a stress-free state, reflecting the tissue’s tone.34 Elevated muscle tone, however, can lead to reduced local blood flow, potentially causing symptoms, such as pain and muscle fatigue.35 36 Dynamic Stiffness: this parameter assesses the tissue’s ability to resist deformation under stress, representing its dynamic elastic properties.34 37 Three parameters related to elasticity are logarithmic decrement, mechanical stress relaxation time and Deborah number. Logarithmic Decrement: this value indicates the degree of energy loss when the soft tissue returns to its original state from deformation and is a dimensionless measure of amplitude decay rate.38 39 Mechanical Stress Relaxation Time: this time parameter reflects the duration required for the soft tissue to return to its initial state after stress is removed.40 Deborah Number: this dimensionless number represents the ratio of deformation time to mechanical stress relaxation time, used to describe the tissue’s recovery ability.41
Muscle fatigue
We will use surface electromyography (sEMG) to analyse muscle fatigue. sEMG is a technique that collects and analyses electrical signals related to muscle fibre contractions by placing electrodes on the surface of soft tissues. The parameters used to measure muscle fatigue include median frequency, mean power frequency and root mean square (RMS). The sEMG signals will be recorded using a six-channel Noraxon Ultium EMG system (Noraxon, USA). sEMG data will be collected using Noraxon Ultium sEMG sensors (Noraxon USA Inc., Scottsdale, AZ, USA) with a sampling frequency of 2000 Hz. The points used for measuring sEMG include: 2 cm lateral to the C5/6 spinous process (splenius capitis), the midpoint between the C7 spinous process and acromion (upper trapezius) and the midpoint between T3 and the medial border of the scapula (middle trapezius).
Before electrode placement, the skin will be prepared by shaving any hair from the measurement sites using a razor and depilatory cream, followed by disinfection with 75% alcohol wipes. The system will be set with a filtering range of 10–500 Hz to remove noise and ensure clean signal acquisition. The output impedance will be set to 10 Ω to minimise the effects of external electrical interference. For normalisation, the maximum RMS value recorded from the same muscle group, with electrodes remaining in place during repeated measurements, will be used as the baseline. Subsequent sEMG readings will be normalised to this maximum RMS value to express muscle activity as a percentage of the maximal recorded value.
Based on pretesting and safety considerations by our team, participants will perform 15 s isometric contractions without any load in both supine and prone positions. Each position will be recorded in two sets, with a 2-min rest period between sets. The dataset with the most comprehensive electromyographic signals will be selected for subsequent feature extraction.
Pressure pain threshold
We will use the OE220 Outcome Evaluation System (ITO, Japan), which comprises an algometer to assess pressure pain threshold. The methodology will follow the user manual and relevant literature. The points used for measuring pressure pain threshold include: 2 cm lateral to the C5/6 spinous process (splenius capitis), the midpoint between the C7 spinous process and acromion (upper trapezius) and the midpoint between T3 and the medial border of the scapula (middle trapezius).42 43
Muscle strength
We will use the OE210 Outcome Evaluation System (ITO, Japan), which comprises a dynamometer, to assess muscle strength. Muscle strength testing will include the measurement of flexion and extension strength. The patient will be seated while the evaluator positions the dynamometer on the patient’s forehead and occipital region. The patient will be instructed to exert force actively, and the evaluator will use the dynamometer to resist the participant’s head, recording the average of three measurements.44 The patient will be instructed to exert force actively, using standardised verbal cues to ensure consistent force application. The evaluator will use the dynamometer to resist the participant’s head, recording the average of three measurements. The patient will be instructed to apply force actively and uniformly, and the evaluator will use the dynamometer to resist the participant’s head, recording three measurements. The mean and SD will be calculated from the three measurements, and the coefficient of variation (CV), defined as the ratio of the SD to the mean, will be computed. If the CV exceeds 20%, the measurement will be considered unreliable, and the test will be repeated.
Data management
Original data will be collected using paper case report forms (CRF) at the designated times. Two data managers, who are blinded to group allocation, will independently receive the completed CRFs and input the raw data into the Epidata system. Both administrators must undergo comprehensive training in data management and data security and will be subject to regular reviews by the DMC. To ensure the protection of patient privacy, the data will undergo deidentification before being stored for further analysis. After data collection is complete, all collected data will be locked, and researchers will not be able to make any modifications to it. The data will be stored for documentation and control purposes for 5 years thereafter, until 31 December 2030.
Statistical analysis
All data will be analysed using statistical software (R 4.3.3/SPSS 29.0). The intention-to-treat analysis set will be used in this study. Multiple imputation techniques will be employed to replace randomly missing data. Normality tests will be performed. Descriptive statistics will be presented as mean±SD for continuous variables that follow a normal distribution and as median (IQR) for those that do not follow a normal distribution, based on the results of normality tests. For ordinal variables, the data will be presented as median (IQR) or frequency (n, %) depending on the nature of the data. Categorical variables will be summarised as n (%).
Changes in repeated outcomes over time will be compared using generalised estimating equations or mixed effects models, with the baseline level of the observed values specified as a covariate. Pearson’s correlation analysis for normally distributed data and Spearman’s correlation analysis for non-normally distributed data, based on the results of normality tests. In other publications, exploratory analyses will include regression analysis, exploratory mediation analysis and other statistical methods, focusing on factors, such as cervical disability index and soft tissue-related parameters.
Adverse events
We will accurately document all adverse events, concomitant medications and concurrent interventions during the clinical trial, rather than prohibiting the use of concomitant medications or concurrent interventions during the trial. Participants who are on long-term medication will continue their usual medication regimen from baseline through follow-up. Any changes in medication and interventions will be recorded by the assessors. Both CFT and Yijinjing training are safe, non-invasive therapies. However, adverse events cannot be entirely ruled out. Possible adverse events include: (1) adverse reactions following exercise interventions, such as increased pain or fatigue; (2) worsening of pre-existing conditions and (3) emergence of new diseases or injuries.
Quality control
Before the trial begins, training will be arranged for all research staff on recruitment, treatment and outcome assessment. Yijinjing practitioners involved in the study must have a professional qualification as a TCM physician or rehabilitation therapist, with at least 3 years of experience in practising Yijinjing and providing rehabilitation therapy. To maintain consistency in the intensity of Yijinjing exercise, all participants will be required to exercise at a moderate intensity for 30 min, defined as 50%–70% of their maximum heart rate (Max HR). The Max HR will be calculated using the formula: Max HR=208–0.7 * age (beats/min).45 For participants with a low fitness level (engaging in less than 150 min of moderate-intensity exercise per week), the target heart rate range will be multiplied by a factor of 0.95. For participants with a high fitness level (engaging in more than 300 min of moderate-intensity exercise per week), the target range will be multiplied by a factor of 1.05. In this study, heart rate and activity intensity will be recorded using Actigraph wGT3X-BT (ActiGraph LLC, Pensacola, FL), worn during each intervention session. To ensure prespecified exercise intensity, participants will wear the Actigraph to measure intensity. Data from the Actigraph will be reviewed after each session, and if necessary, the treating physician will adjust the participants’ exercise intensity, including modifying the number and range of movements, to achieve the prespecified intensity. To ensure that participants complete their home practice, they will be asked to practise at home via WeChat group video at 6 PM, under video supervision. A practice log will be provided to participants, which they are required to complete after each home exercise session. If participants forget to complete the log, they will be encouraged during the following session to recall and record the details of their home training activities. Instances where training is not performed or does not meet the prescribed intensity will be recorded. Participants who complete at least 20 sessions at the prescribed intensity will be classified as having high adherence, those completing 12–19 sessions will be categorised as having moderate adherence, and those completing fewer than 12 sessions will be considered to have low adherence. If necessary, further analyses will be conducted based on adherence levels.
During the study, an independent DMC will be established to review the adherence to standardised procedures. The DMC will consist of two independent musculoskeletal disease experts and one statistician who is not involved in the project. The DMC will meet every 3 months to review enrolment status, study procedures, completion of CRF, data quality, follow-up losses and safety data results. In the event of a serious adverse event, the DMC will convene to decide whether to terminate the study early.
Ethics and dissemination
Ethical approval for this study was obtained from the following ethics committees: the Ethics Committee of Shanghai Municipal Hospital of TCM (approval number: 2024SHL-KY-91–01), the Ethics Committee of Shuguang Hospital affiliated to SHUTCM (approval number: 2024-1617-200-01) and the Ethics Committee of Yueyang Hospital of Integrated TCM and Western Medicine affiliated to SHUTCM (approval number: 2024–210). The study will adhere to the principles outlined in the Declaration of Helsinki. It will also comply with local laws, regulations and the policies of the ethics committees at each centre.
The trial will officially commence only after obtaining written informed consent from all participants. Data collected during the trial will be used solely for this clinical research. Participants’ privacy will be protected, and their personal information will not be disclosed. It will not be possible to identify individual participants from the information provided in the disseminated results. The results of the study will be published in peer-reviewed journals or presented at academic conferences.
Discussion
This study is an innovative, multicentre, RCT designed to compare the clinical efficacy of mind–body exercise and CFT in patients with NCNP. Given that the primary symptom of NCNP is neck pain, the main objective of this study is to assess the difference in effectiveness between the two exercise programmes in alleviating neck pain. Additionally, the study aims to evaluate the impact of the exercise programmes on cervical function, range of motion and musculoskeletal and soft tissue status as secondary outcomes. We anticipate that the intervention group will show a significant reduction in pain levels and achieve better improvements in cervical function, range of motion and muscle status.
Musculoskeletal disorders are often caused by mechanical injuries to soft tissues. During the repair and remodelling process following soft tissue damage, immune cells participate in clearing the damaged tissue, accompanied by a persistent inflammatory response.46 Continuous mechanical injury can lead to chronic inflammation, lowering the pain threshold and triggering pain perception.47 Additionally, the balance of soft tissue repair is disrupted, leading to excessive proliferation of fibroblasts and alterations in the structure and physical properties of the soft tissue.48–50 Biological research shows that exercise can enhance the self-protective capacity of soft tissues and reduce various stress responses caused by stressors.51 52 Exercise training has been found to promote the secretion of myokines by muscle tissue, which can inhibit the production of the proinflammatory cytokine TNF, such as TNF-α, while stimulating the release of anti-inflammatory cytokines, such as interleukin-1ra (IL-1ra) and interleukin-10 (IL-10), thereby exerting an anti-inflammatory effect.53 A review has shown that some clinical studies have found that mind–body exercises promote a reduction in inflammatory markers, such as C reactive protein and IL-6, as well as a decrease in the signalling of the proinflammatory transcription factor nuclear factor kappa B.54 Additionally, moderate exercise may enhance glycosaminoglycan content in articular cartilage, contributing to the mitigation of joint degeneration.55
Recent studies have shown that functional training can improve the efficacy of pain relief.56–59 A Cochrane systematic review provides moderate evidence that neck strength training, neck stretching and neck stability training can alleviate chronic neck pain.60 Additionally, previous meta-analyses have indicated that core stability training yields greater pain relief for chronic low back pain compared with general exercise.61 Some prior neck exercise protocols have also incorporated core stability movements.10 Considering the potential benefits of core stability training for NCNP, we have included core stability exercises in our CFT protocol to ensure optimal efficacy.
A review of exercise therapy for musculoskeletal disorders indicates that exercise training can improve pain, functional disability, muscle strength and balance.9 56 A meta-analysis on chronic neck pain further supports that exercise training effectively alleviates pain and functional disability.11 62 Our previous research demonstrated that exercise combined with Tuina can reduce soft tissue stiffness.14 These findings suggest that exercise therapy has potential efficacy in improving pain, functional disability, muscle stiffness and muscle strength in patients with NCNP. Therefore, the CFT programme was comprehensively designed to include key elements, such as stretching exercises, strength training and stability training, ensuring a holistic approach to addressing cervical function and overall rehabilitation needs. The training movements were adapted from the corrective exercise guidelines of the NASM and relevant literature to ensure the effectiveness of the rehabilitation treatment.10 25 To improve patient adherence and facilitate safe, independent training during follow-up, we incorporated resistance bands and small exercise balls into the CFT. Yijinjing is currently primarily used for health education rather than specific exercise guidance in the guidelines.14 63–66 Recent studies have investigated the use of Yijinjing in treating various musculoskeletal conditions, including NCNP and rheumatoid arthritis (RA).14 67 68 Preliminary research indicates that Yijinjing is effective as a complementary therapy in reducing pain levels in patients with NCNP and in improving hand function in individuals with RA.67 These findings support the potential of Yijinjing as a therapeutic exercise for various musculoskeletal conditions, particularly in reducing pain and improving function in the management of NCNP.14 Further research is needed to elucidate its efficacy in other musculoskeletal conditions. Yijinjing incorporates exercise methods similar to functional training, such as simultaneous stretching and stability training, coordinated cervical and thoracic spine movements and bodyweight exercises to enhance neck strength, but its clinical application remains focused on promoting general health and wellness rather than being a prescriptive regimen in clinical guidelines. Based on the available evidence, it is reasonable to hypothesise that Yijinjing, as an independent treatment, may have a significant impact on alleviating pain, improving function and enhancing soft tissue conditions in the management of NCNP. Given that Yijinjing consists of specific bodyweight exercises that are not constrained by the environment or equipment, and that its movements are gentle and less likely to cause injury, it is reasonable to expect potential benefits from this approach.
During the implementation of the trial, several potential issues may arise, including exercise-induced injuries, missed intervention guidance, loss of follow-up, participants neglecting the risks of neck pain due to temporary pain relief, resulting in incomplete exercise training or abandoning training due to a lack of perceived improvement. To mitigate these risks, we have implemented several measures early in the trial. (1) Participant Selection: we have established strict inclusion and exclusion criteria to select theoretically suitable participants and emphasised the importance of regular exercise training. We also excluded individuals unsuitable for exercise training. (2) Communication: participants are notified in advance about treatment, assessments and follow-ups via SMS or email. We provide our contact information to prevent data incompleteness due to participant dropout. (3) Monitoring and Intervention: if symptoms worsen or if other conditions make continued exercise training unsuitable, we will immediately halt the intervention, and the DMC will decide whether to remove the participant from the study. (4) Medical Access: we offer multiple avenues for participants to access medical services to ensure that they receive timely healthcare, thereby reducing risk.
Yijinjing is easy to learn and self-administered, and it is not limited by time or space. If shown to improve clinical symptoms inpatients with NCNP and to be as effective as CFT, it could not only justify its promotion within the community but also support the recognition of mind–body exercises as a self-management method in clinical guidelines, particularly in environments lacking structured rehabilitationprogrammes. This may encourage their broader incorporation into future rehabilitation practices, providing a more diverse range of exercise training options forpatientswith NCNP. Such findings would also assess whether Yijinjing offers superior effects on pain, functional impairmentand soft tissue degenerationcompared with other treatments. In future research, we plan tocompareYijinjing with commonly used no-equipment exercise programmes, such as McKenzie exercises, as well as with other mind–body exercises.69 ,70 This will help provide insights into the efficacy and cost-effectiveness of different exercise interventions, offering valuable guidance for selecting low-cost exercise options for clinical practice.
Ethics statements
Patient consent for publication
Acknowledgments
We thank all the investigators, study staff, outcome assessors and participants for their support in this trial.
References
Footnotes
Contributors Guarantor is FY. HZ and FX contributed to the concept and design of the study. HZ and FX developed the hypothesis and wrote the original proposal. HZ, FX, HY, XC and ZW significantly contributed to the creation of the manuscript. MF and FY performed critical revisions of the manuscript. HZ and FX drafted the manuscript. All authors reviewed and approved the final version of the manuscript. I used ChatGPT to refine the language in the manuscript, improving its overall coherence and fluency.
Funding Shanghai Key Laboratory of Traditional Chinese Medicine Tuina Techniques for Musculoskeletal Diseases (24dz2260200); The National Natural Science Foundation of China (82474672); The Future Plan for Traditional Chinese Medicine Development of Science and Technology of Shanghai Municipal Hospital of Traditional Chinese Medicine (WL-JXXK-2021001 K).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.