Article Text
Abstract
Introduction Weight loss is often recommended as the primary intervention for infertility in individuals with high body mass index. However, focusing on body mass index might overlook other important factors like cardiometabolic health. This study aims to examine cardiometabolic health in patients seeking fertility treatment and its impact on reproductive outcomes.
Methods and analysis A cross-sectional analysis of 800 systematically selected participants (400 couples) will be completed on a single visit to the study site. This session will involve taking blood samples to examine metabolic biomarkers, the completion of questionnaires assessing preconception health factors and an exercise ‘step test’ to assess cardiorespiratory fitness. Metabolic panels will be compared with target values and, where available, normative population data. Fitness data will be transformed into normative percentile values based on the participant’s age and sex. Patients will be followed for 2 years to allow yearly data collection related to conception, gestation and parturition. Associations between cardiometabolic health during the preconception phase and reproductive outcomes will be examined.
Ethics and dissemination The Newfoundland and Labrador Health Research Ethics Board has provided ethical approval for this study (HREB #20230825). Each patient will be required to give written consent prior to any data collection. We will share study findings at conferences and submit manuscripts to peer-reviewed journals. Additionally, we will create knowledge translation presentations for Newfoundland and Labrador Fertility Services and Family Practice Clinics.
- Reproductive Medicine
- Subfertility
- General endocrinology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
A clinically comprehensive and accessible set of assessments associated with cardiometabolic health for couples seeking fertility treatment is proposed.
Patients will be followed longitudinally to identify associations between cardiometabolic health during the waiting period and reproductive health outcomes.
Recruiting participants for exercise testing can be challenging, as it may be viewed as burdensome by some individuals.
This single-site study faces challenges due to the province’s vast geography, as transportation and travel time may hinder participation for some patients.
Introduction
Over the past 30 years, infertility rates have risen significantly and now affect one in six Canadian couples.1 Alongside the increased prevalence of infertility, obesity has become a growing health issue. In 2018, 40.2% of Newfoundland and Labrador (NL) residents aged 18 and older were classified as obese.2 The rates of obesity (BMI>30 kg/m2) for women of childbearing age (25–34 years) have tripled in NL over the past three decades. This poses a considerable challenge to fertility, as women with obesity are three times more likely to experience infertility compared with those with a normal BMI (18.5–24.9 kg/m2).3 Increasing obesity rates have also significantly impacted male fertility. In Canada, the proportion of obese adults is higher among males (69.4%) than females (56.7%) for all age groups starting at age 20.2 4 The prevalence of obesity among reproductive-aged men has steadily increased over the past few decades with a concurrent decline in male fertility rates.5
Due to the known complications associated with obesity and reproductive health, weight loss is the primary recommended non-pharmacological intervention for individuals with elevated body mass index (BMI) and infertility.6 Patients with a BMI greater than 25 kg/m2 are advised to reduce their body weight by 5%–10%.7–10 Advising patients to lose weight without the proper support has negative consequences. For example, patients may experience adverse health outcomes to non-patient-centred approaches to weight loss counselling, such as medication non-adherence, mistrust of their healthcare provider and avoidance of medical care.11–13 Additionally, a potential repercussion of hyperfocus on weight loss is that using BMI as the gold standard for managing obesity and infertility has diminished the importance of assessing other biophysical markers associated with cardiometabolic health.14
Cardiorespiratory fitness is a key indicator in routine health assessments and has been associated with improved overall health and reduced mortality risk.15 16 Pregnancy is known as the ‘stress test for life’ and increases the resting heart rate (RHR) by approximately 20% and cardiac output by 30%.15 Adequate cardiorespiratory function supports the increased physiological demands placed on the body during pregnancy. Moreover, maintaining a healthy level of cardiorespiratory fitness has been associated with reduced risks of hypertensive disorders such as pre-existing hypertension, gestational hypertension, pre-eclampsia and gestational diabetes during pregnancy.15 Therefore, cardiorespiratory fitness is a crucial component of reproductive health outcomes, as it ensures the body’s ability to meet the physiological demands of pregnancy and reduces the risk of pregnancy-related complications. However, limited research has explored the cardiorespiratory profile of fertility patients and its implications for reproductive health outcomes. Thus, rather than assessing BMI as the first line of evaluation, emphasising improving markers of cardiometabolic health through increased physical activity and reduced sedentary behaviour may be more beneficial.
In NL, within 6 months, there are approximately 319 patients on an approximately year-long waiting list to see a fertility physician at the Newfoundland and Labrador Fertility Services (NLFS). This ‘waiting period’ not only increases patients’ anxiety and stress, known to impact outcomes related to fertility, but maternal extended periods of waiting can also worsen an infertility prognosis.17 To enhance the quality of fertility care and research, we propose a study protocol to evaluate cardiometabolic markers and their link to fertility and pregnancy outcomes. This research could improve healthcare for individuals seeking fertility services, leading to better fertility and pregnancy outcomes and ultimately enhancing the overall well-being of patients on the NLFS waitlist.
Study objectives
This study aims to evaluate cardiometabolic markers during the preconception phase that may impact health outcomes throughout conception, pregnancy and postpartum. By assessing couples’ metabolic and cardiorespiratory profiles while they await fertility services, we will explore the association between these markers (eg, fasting glucose, lipid profiles, predicted VO₂ max and physical activity levels) and reproductive health outcomes. Specifically, the study goals are to: (1) compare participants’ cardiorespiratory fitness with established normative data based on age and sex; (2) compare metabolic panels with target values and available normative data; (3) examine the relationship between cardiorespiratory fitness, the Metabolic Syndrome Severity Score (MetSSS) and fertility and pregnancy outcomes, such as live birth and (4) report patients’ knowledge of preconception risk factors to guide future research.
Methods
Study design
The data collection procedure for this study follows a prospective cohort study design. On enrolment in the study, individuals will provide informed consent prior to any data collection. All data will be collected during a single visit. The participant’s data will be collected and linked to a non-identifiable participant ID to ensure confidentiality throughout the research process. There will be a master list linking the participant ID number to participant names, but this list will only be accessible to fertility physicians and research leads. The master list will be stored separately from other study data in a password-protected file in a locked cabinet. There will be no interventions or manipulation of variables during the study. Table 1 and figure 1 detail the schedule of enrolment and assessments for data collection.
Schedule of enrolment, assessments and personnel responsibilities
Flow chart of recruitment and data collection procedures. *mCAFT, modified Canadian Aerobic Fitness Test.
Study setting
Data collection will occur at the Department of Research and Innovation, which is a centre embedded within the provincial health authority in St. Johns, Newfoundland and Labrador, Canada.
Characterisation of waitlist population
To determine the feasibility of the present study’s protocol, the research team sought to characterise the waitlist population over a 6 month period. In 2023, the NLFS implemented a standardised form outlining 11 distinct reasons for referral (see online supplemental appendix 1). Referral forms to the NLFS that were reviewed by the fertility physicians were removed from any directly identifiable information. Each patient was assigned a participant ID to ensure confidentiality. During this period, 221 patients were referred for infertility (including an inability to conceive, ovarian insufficiency or abnormal seminal analysis).
Supplemental material
Sample size
We estimated the minimum required sample size for a logistic regression model to predict live birth, conception method (ie, assisted/unassisted) and time-to-pregnancy (TTP). A 20% prevalence of assisted pregnancy was assumed, with nine predictor variables, a c-statistic of 0.8 (indicating moderate to high discrimination) and a shrinkage factor of 0.9. The estimation indicated that a minimum of 400 couples are needed.
Sampling strategy
Patients referred to the fertility clinic over a 6 month period will be placed on a waitlist, which will be pseudo-randomly sorted alphabetically. A random starting position on the list will then be selected, and every nth patient will be chosen according to a calculated sample interval. The sample interval is determined by dividing the total number of patients on the waitlist by the desired number of patients to be sampled. If the end of the list is reached before the required number of patients is selected, the process will wrap around to the start of the list and continue until the original starting position is reached. This process will be repeated for four consecutive 6 month periods. While the number of patients may vary in each period, 100 patients will be sampled per period to reach our target of 400 couples within 2 years of data collection. Recruitment for the study will begin in March 2025 and will be completed by March 2027.
Inclusion and exclusion criteria
The inclusion criteria for this study are as follows: (1) interest in participating in the research; (2) at least 18 years of age and below the age of 45 at the time of consent and (3) referral to the NLFS for infertility. Participants will be excluded from the research if they have physical limitations that would prevent them from safely completing the fitness assessment portion of the study and have a BMI under 18.
Informed consent
Each patient will be required to give written consent prior to any data collection. Following the systematic sampling, chosen participants will receive a letter from NLFS informing them of their selection for potential participation in the study. The letter will indicate that the selected individual and their partners are encouraged to contact the research team if they are interested in participating. On expression of interest, participants will receive a detailed description of the study from a research member. If they remain interested in participating, they will be provided with the consent form via email or mail, allowing them to review it before the initial visit. During the data collection session, the research team will review the study procedures and the informed consent documents with the participants. Participants will be given time to review the consent forms, ask questions and receive clarification on any aspects of the study before signing any documents.
Data collection, management and analysis
Patients will attend one visit for data collection that will, on average, take approximately 2.5 hours. Patients’ information will be collected, including (1) age, height, waist circumference, weight and blood pressure, (2) list of medications, (3) medical history (eg, cardiovascular disease and/or diabetes), (4) duration of infertility (including whether it is primary or secondary), (5) relevant obstetrical history (including previous pregnancies, complications in pregnancies, recurrent pregnancy loss and postpartum complications) and (6) if the cause of infertility in women and men is known.
Preconception metabolic profile
During the data collection session, blood samples will be obtained to examine metabolic panels. A registered nurse will draw blood samples from each participant during the session. Participants will be instructed to fast for 8 hours before the blood draw. The blood samples will be sent to the provincial health authority’s clinical laboratories, where they will be analysed for fasting glucose and lipid profiles.
Metabolic Syndrome Severity Score: metabolic syndrome is a group of risk factors for cardiovascular disease and diabetes that often occur together. MetSSS was developed to support clinical diagnosis and treatment decisions.18 The MetSSS will be calculated from the following collected measurements: sex (female/male), systolic blood pressure (mm/Hg), diastolic blood pressure (mm/Hg), triglycerides (mmol/L), high-density lipoprotein cholesterol (HDL-C) (mmol/L), blood glucose (mmol/L) and waist circumference (cm).18
If the participant consents, the research team will review the blood work results for any concerning findings, such as diabetes, high blood sugar, elevated cholesterol or abnormal lipid levels. If any issues are identified, a physician from the research team will follow-up with the patient in the fertility services clinic or refer them to the appropriate healthcare provider for further evaluation and management. To maintain confidentiality, participants’ samples will be labelled with their assigned participant ID. Any remaining samples after testing will be destroyed.
Preconception behavioural questionnaires
We will use the Preconception Health Knowledge Questionnaire (PHKQ) to assess preconception risk factors such as patients’ reproductive history, sexual health, infectious diseases, chronic medical conditions, mental health, medications, immunisations, lifestyle behaviours, psychosocial stressors and environmental exposures. The PHKQ is comprised of 25 multiple-choice questions and takes approximately 5–10 min to complete.19
Preconception cardiorespiratory fitness profile
We will administer the Get Active Questionnaire from the Canadian Society for Exercise Physiology (CSEP). This self-administered questionnaire will assess the participant’s health and medical history as a prescreening for exercise testing. If any health conditions or risk factors are identified through this questionnaire, participants will be referred to their healthcare provider for further evaluation and guidance before participating in the study’s exercise testing component. Additionally, to collect patients’ physical activity history, the Godin Leisure-Time Exercise Questionnaire will be administered to evaluate leisure-time physical activity, focusing on the frequency and intensity of these activities.20
RHR and resting blood pressure (RBP) will also be measured to gauge participants’ readiness for exercise testing. If the participant’s RHR is found to be ≥100 beats per minute after a second reading or their RBP measures≥160/90 mm Hg after two readings, they will not proceed to the exercise testing portion of the study due to potential health risks. However, if a participant’s resting systolic BP falls between 140 and 160 mm Hg, and they have not been diagnosed with hypertension, they will be eligible to participate in the exercise assessment.21
Participants’ cardiorespiratory fitness will be assessed through a standardised exercise ‘step test’ known as the modified Canadian Aerobic Fitness Test (mCAFT), which adheres to CSEP guidelines.22 The mCAFT involves stepping on and off a step platform for a predetermined duration while maintaining a stepping cadence. A qualified Kinesiologist will administer the test and continuously monitor participants’ heart rate and rating of perceived exertion throughout. The test will end when the participant reaches 85% of their heart rate maximum, or another test termination criterion occurs, such as the participant asking to stop, showing signs of physiological distress or not maintaining the cadence of the test. From this test, the predicted maximum rate of oxygen uptake during exercise (VO2 max) will be calculated using the following equation:23
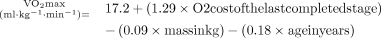
Longitudinal data on fertility and pregnancy-related outcomes
Participants will be followed for 2 years on the NLFS waiting list, and medical data will be gathered annually on the conception, prenatal and postpartum phases of reproduction. We will examine variables documented in both the NLFS and provincial perinatal databases. The primary outcome of this study is live birth, which is defined as delivery after ≥20 weeks gestation and information including gestational age, birth weight and sex of infant.24 Secondary outcomes include TTP and the method of conception (ie, assisted or unassisted). Additional predictors that will be used to describe the cohort will include reproductive health conditions (eg, ovulatory disorders (eg, thyroid dysfunction, hyperprolactinaemia, polycystic ovary syndrome (PCOS), hypothalamic amenorrhoea, female-other (eg, tubal occlusion, endometriosis, diminished ovarian reserve and uterine factors) and/or male factor).
Adverse events
Throughout the study, participants may encounter potential adverse events. Participants face a moderate risk of falling during the fitness assessment. While rare, a cardiac event can also occur during fitness testing. However, precautions will be taken to minimise this risk through supervision by trained personnel and screening measures to identify participants at higher risk. Participants may experience discomfort, anxiety, emotional and/or psychological distress while completing questionnaires due to the nature of the questions asked. Participants may take a break, skip or stop answering questions at any time, and resources will be provided to manage any distress experienced. There is also a possibility of pain, bruising, swelling or infection at the blood draw site and/or discomfort from needle insertion. Proper sterilisation techniques and trained personnel will be employed to minimise these risks. Despite protections, there is also a risk of unintentional release of healthcare information. Participants will be advised to report adverse events to the research team. We will document the number of participants who withdraw from the study due to a related adverse event and those who experience one or more related adverse events and their types.
Data management
All research team members will complete and maintain training on privacy protection and research ethics and will sign a confidentiality agreement. All computer files related to the study will be password-protected, and all data collected during the study will be kept at the data collection site and stored in a password-protected file in a locked cabinet in a locked office. Data and study samples will be labelled with a unique identifier (eg, WFS000). This identifier will not be associated with the participant’s personal information, such as name or date of birth. The only research team members with access to the identifiable data are research leads, the phlebotomist performing the blood draw, NLFS physicians and the research nurse. Electronic data will be securely erased to ensure that no residual information remains on the device or storage components before disposal or repurposing of the system. Data and samples will not be used for future research and will be destroyed 5 years after the study’s close date. Data will be available in a public, open-access repository.
Statistical analysis
The study will use descriptive and correlational analyses to provide a comprehensive overview of the participants’ characteristics and the distribution of cardiorespiratory fitness levels and metabolic measures. First, to compare participant cardiorespiratory fitness levels with established normative data, individual scores will be converted to sex and age-specific z-scores. A one-sample t-test will be used to statistically test for significant differences between participant fitness scores and normative data. Similarly, metabolic panels will be compared with target values and, where available, normative population data (based on age and sex).
To examine the relationship between our cardiometabolic predictors, (1) cardiorespiratory fitness (maternal and paternal scores) and (2) MetSSS (maternal and paternal scores) and (3) reproductive health diagnoses (ovulatory disorders (eg, thyroid dysfunction, hyperprolactinaemia, PCOS, hypothalamic amenorrhoea and female-other (eg, tubal occlusion, endometriosis, diminished ovarian reserve, uterine factors) and/or male factor) and our primary outcomes of interest (eg, live birth, serum pregnancy, ongoing pregnancy (≥12 weeks), etc), both multiple linear regression and multiple logistic regression will be used, depending on whether the outcome variables are continuous or binary. For our secondary outcomes, we will examine TTP using a Cox proportional hazards regression model with cardiometabolic and reproductive health diagnoses predictors. Further logistic regression analyses will be used to examine ‘assisted’ versus ‘unassisted’ conception with respect to the cardiometabolic and reproductive health diagnoses predictors. Each regression model will be adjusted for maternal and paternal age.
Potential limitations of the research plan
We have determined our sample size (n=800 participants, 400 couples) based on our proposed statistical analysis. However, challenges may arise during the recruitment phase, potentially impacting the ability to attain the desired sample size. Recruiting participants to partake in exercise testing is challenging, as it often requires a time commitment and may be perceived as burdensome by some individuals. Additionally, our research faces a unique challenge due to the expansive geography of our province. Transportation and travel time may pose logistical challenges for patients outside of the city, where data collection will occur, potentially impacting their ability to participate in the study.
Sex and gender considerations
Sex as a biological variable will be accounted for throughout the research process. The research team recognises that infertility affects both males and females, and we recognise the importance of inclusivity in the research process. Therefore, we aim to investigate the experience and cardiometabolic markers of both males and females affected by infertility. Furthermore, we recognise that gender is a sociocultural determinant of health. Participants will be asked to self-identify their gender to address these effects and mitigate gender-related biases. Gender is considered throughout the research process, including the literature review, methods, recruitment, analysis, implementation and knowledge translation plan.
Patient and public involvement
Throughout the planning and implementation of this study, engagement with patient partners has been central to ensuring that the research remains grounded in the lived experiences and needs of individuals and couples affected by infertility. Their insights have helped to ensure that the study is relevant and sensitive to the diverse perspectives of patients on the waiting list for fertility services in NL. Their continued involvement will assist us in the dissemination of research findings, identifying implications for clinical practice and advocating for patient-centred reproductive care in the province.
Ethics and dissemination
We will disseminate study findings through local, national and international conference presentations, and we plan on submitting multiple manuscripts for publication in peer-reviewed journals. We will also create knowledge translation presentations with patient partners for the NLFS and Family Practice Clinics. Furthermore, we will develop community-based presentations with patient partners for interested members of the public and fertility patients. The Newfoundland and Labrador Health Research Ethics Board has approved this study (HREB #20230825).
Significance of research
This study is an essential step in characterising the spectrum of profiles of patients with infertility and the influence of modifiable risk factors on reproductive health. The impact of this research extends far beyond the immediate findings, holding the potential to reshape the landscape of fertility care. In recent years, the province of NL has seen a rise in patient advocacy groups demanding better quality fertility care. By exploring the often-overlooked link between cardiorespiratory fitness and reproductive health outcomes, our research aims to introduce personalised interventions and advocate for a shift in preconception care. This shift could significantly enhance fertility outcomes and the overall well-being of individuals on the NLFS waitlist.
Ethics statements
Patient consent for publication
References
Footnotes
X @LaurieTwells
Contributors HM, HMM and KW contributed to the writing of the manuscript. LT, KW, ELM, FAB, EWR and JC contributed to the design of the research protocol. LT, KW, ELM, SH, DM, SM and FAB conceived the original idea. All authors contributed to the reviewing process. KW is the guarantor.
Funding This work was supported by the Dean’s Collaborative Grant from the Faculty of Medicine, Memorial University of Newfoundland, grant number 215938.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.



