Article Text
Abstract
Objective To assess the association of C-reactive protein (CRP) with postoperative infections for eight different types of surgery using big data.
Design A multicentre cohort study with longitudinally collected data from electronic health records, collected from 1 January 2011 to 22 September 2023.
Setting Data of two tertiary medical centres in the Netherlands were used.
Participants This study included all procedures (42 125 in total) in adult patients undergoing surgery in two tertiary medical centres in the Netherlands.
Outcome measures The primary outcome was the association between CRP and a postoperative infection in the first 30 days postoperatively. Postoperative infection was defined by an action-based definition, that is, patients had to be treated for an infection with anti-microbial treatment and/or an intervention (eg, surgical drainage) to be classified as having a postoperative infection. CRP measurements were divided into a reference group (0–5.0 mg/dL) and four groups for comparison (5.1–10.0 mg/dL, 10.1–15.0 mg/dL, 15.1–20.0 mg/dL and >20.0 mg/dL). Subgroup analyses were performed for eight major surgical subspecialties and for the two medical centres separately.
Results A total of 175,779 CRP measurements were performed, of which the majority was drawn in the first postoperative week. The ORs for developing a postoperative infection varied between 1.0 (0.9–1.1 95% CI) and 12.0 (9.5–15.1 95% CI), with a stronger association for the higher level of CRP categories and when more time had elapsed since surgery. Sensitivity ranged between 11% and 34%, specificity ranged between 64 and 95%, and the positive and negative predicting value ranged between 12% and 51% and 88% and 94%, respectively. For the surgical subspecialties and the two hospitals separately, similar results were found.
Conclusion In this study, an elevated postoperative CRP was associated with postoperative infections with a stronger association for higher CRP levels. The association was stronger if a longer time had elapsed since surgery, which contrasts with the moment most CRP measurements were done, namely in the first postoperative week. Clinicians should take the evolving value of CRP in mind when using it in the diagnosis of postoperative infections.
- Adult surgery
- Molecular diagnostics
- Clinical Decision-Making
- Observational Study
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The cohort consisted of a large, ‘real world’ sample of adults from two different academic hospitals.
A clinical action-based definition for postoperative infection was used and tested on a random sample of patients with good correspondence.
To prevent CRP measurements in patients with a beginning infection being counted in the non-infection group, patients were excluded from the non-infection group 1 week before the start of infection treatment, as the exact start of infection could not precisely be determined.
Introduction
More than 300 million surgical procedures are performed worldwide each year.1 It is estimated that 6.5 to 18 percent of all patients undergoing surgery will develop a postoperative infection in the first 30 postoperative days.2–5 A large proportion of infections is diagnosed after the eighth postoperative day and increasingly after discharge from the hospital.6 7 Early diagnosis and treatment are essential to prevent further deterioration of the clinical condition of the patient. Moreover, unnecessary treatment with antibiotics or a reintervention should be avoided. A wide array of serum biomarkers and prediction models have been used to discriminate between patients with and without a postoperative infection. The most widely available and used marker for this purpose is C-reactive protein (CRP).
CRP is an acute phase protein, produced in the liver in case of inflammation or infection in response to pro-inflammatory cytokines.8 9 CRP levels are elevated during the first postoperative days due to tissue damage caused by the surgery itself, with its peak around the third postoperative day.9 10 After these first days, CRP slowly declines to its baseline values. Consequently, a high CRP in the first postoperative days often causes a clinical dilemma: it is either an elevated CRP related to the surgical intervention or a first sign of infection.
This knowledge gap still exists as meta-analyses have shown different discriminative accuracies of CRP in patients who underwent surgery, with a C-statistic varying between 0.66 and 1.00.11–13 These variations in predictive ability may be explained by differences in selected cutoff values, postoperative day of measurement, type of surgery and the type of predicted infection.11–13 Moreover, most studies have focused on CRP levels solely in the first postoperative week, included only a small number of patients and used different diagnostic criteria for postoperative infection. Therefore, we have analysed the CRP data from a large multicentre cohort of postoperative patients, with a follow-up of 30 days and with the use of a standardised definition for postoperative infection. The aim of this study was to obtain insight into the clinical use of CRP and its potential diagnostic value as a biomarker for the diagnosis of any type of postoperative infection.
Methods
Study design and population
We conducted a cohort study with the use of electronic health record databases as part of the PERISCOPE project.14 The PERSISCOPE study aims to develop, validate and locally retrain a machine learning algorithm for the prediction of postoperative infections with the use of existing data from electronic health records.14
The databases include detailed information about 158 703 procedures in adult patients (age ≥18 years) that underwent surgery in two large tertiary medical centres in the Netherlands (the Leiden University Medical Center (LUMC) and the Radboud University Medical Center Nijmegen (RadboudUMC)) between 1 January 2011 and 22 September 2023 (see online supplemental etable 1 in the Supplement for a full list of data types used from the databases). Procedures from eight surgical subspecialties (general surgery, cardiothoracic surgery, neurosurgery, urological surgery, orthopaedic surgery, gynaecological surgery, ear-nose-throat (ENT) surgery and maxillofacial surgery) were included. Patients could be included more than once when they underwent multiple surgeries within the study period. Non-invasive procedures (eg, anaesthesiologic or biopsies) were excluded, as well as re-operations within 30 days of the previous surgery.
Supplemental material
As there is under-registration of complications in real-life clinical practice,15 a clinical action-based definition of postoperative infection was used in which postoperative infections were defined as the start of non-prophylactic antibiotics (initiated >24 hours postoperatively and with a minimum duration of 72 hours) and/or a surgical intervention for an infection such as drainage and re-operation within 30 days of the index surgery. All types of postoperative infections were included (see online supplemental etable 2 in the Supplement for the full definition used for postoperative infection). All CRP values measured up to 30 days postoperatively were included. Patients without any CRP measurement in the postoperative period or patients with a possible preoperative infection based on the surgical procedure (manually checked with the use of ICD10 codes and other, hospital specific, diagnosis codes) or a preoperative CRP >2.5 mg/dL in the 5 days preceding the operative procedure were excluded from all analyses.
Statistical analysis
To analyse the CRP results, patients were divided into two groups based on their infection status for each postoperative week separately. CRP measurements from patients who developed a postoperative infection within 30 days of surgery were included in the group without an infection until 1 week before developing the postoperative infection, as the precise moment of start of the infection could not be determined, retrospectively. In the postoperative infection group, only CRP measurements from the 24 hours before and after the start of treatment were included for analyses (online supplemental efigure 1 in the Supplement). If multiple CRP values of one patient on the same day were available, the maximum CRP value for that day was used.
Descriptive statistics were used for baseline characteristics. Continuous variables were presented as mean and SD or median and IQR, as appropriate. Categorical variables were reported as absolute numbers and percentages. The Mann Whitney U test was used for continuous variables as data were not normally distributed. The χ2 test was used for categorical variables. ORs with 95% CIs, sensitivity, specificity and negative (NPVs) and positive predictive values (PPVs) were calculated to examine the strength of the association between postoperative infection and CRP (per stratum: 5.1–10.0 mg/dL, 10.1–15.0 mg/dL, 15.1–20.0 mg/dL and >20.0 mg/dL) per postoperative week. The CRP range of 0–5.0 mg/dL was used as the reference stratum. The stratification of CRP by 5 mg/dL was based on consultation of different clinical specialists and clinical expert discussion. A subgroup analysis was performed for the different major surgical subspecialties and for the two hospitals separately. We judged that most of the time, CRP was measured for a reason. Thus, missing CRP data were not missing at random and were therefore not imputed.
All statistical analyses were performed in Python (Python Software Foundation, Beaverton USA, V.3.8).
Ethics
The study was approved by the Medical Ethics Assessment Committee of the LUMC (METC-LDD (Medisch Ethische Toetsingscommissie Leiden-Den Haag-Delft)) and RadboudUMC (METC Oost-Nederland) protocol nr G18.129; the research performed with the data complied to the Dutch legislation, the declaration of Helsinki and good clinical practice. Informed consent was not required as this was a database study with anonymised data.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
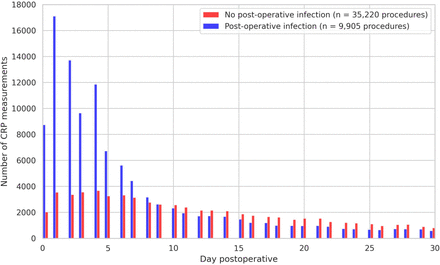
Of the 158 703 procedures in the database, a total of 45 125 surgical procedures from 40 009 unique patients were included in the study. 113 578 procedures were excluded because they were either a non-invasive procedure (n=54 461), re-operation within the 30-day postoperative period (n=12 649), patients did not have a recorded CRP measurement in the 30-day postoperative period (n=41 125) or because patients had an elevated CRP (>2.5 mg/dL) in the preoperative period (n=5343). During the first 30 days postoperatively, 175 779 CRP measurements were recorded, of which the majority (n=1 07 002; 61%) was requested in the first week (figure 1). Finally, for patients with more than one CRP measurement per day, the maximum value per day was included for the analyses, excluding 4988 CRP measurements. Therefore, of the 175 779 CRP measurements, 170 791 measurements were included in the final analyses.
Absolute numbers of C-reactive protein (CRP) measurements in the first 30 postoperative days for patients with and without infection.
In 9905 (22%) of the procedures, a postoperative infection was present. Postoperative infections occurred more often in male patients and in non-elective procedures. All baseline characteristics of the included procedures are summarised in table 1. CRP levels in patients with and without a postoperative infection were almost similar in the first days postoperatively. After the first week, patients with a postoperative infection more often had an elevated CRP and a higher CRP compared with patients without a postoperative infection (figure 2). The OR for developing a postoperative infection increased with higher CRP category and a longer time elapsed since surgery. The ORs varied between 1.0 (CRP 5.1–10.0 mg/dL in the first postoperative week) and 12.0 (CRP >20.0 mg/dL in the third postoperative week) (figure 3, online supplemental etable 3). Sensitivity was low for all weeks and CRP value categories, ranging between 11% and 34%. Specificity ranged between 64% and 96% and increased with higher CRP categories and a longer time since surgery. The PPV of CRP ranged between 12% and 51%. The NPV of a CRP ≤5.0 mg/dL ranged between 88% and 94% (online supplemental etable 3).
Descriptive characteristics of included surgical procedures
Distribution of incremental C-reactive protein (CRP)-level strata for patients with or without a postoperative infection per level of CRP over time. (A) measurements for patients that had no infection in the first 30 postoperative days. (B) Measurements for patients with an infection in the first 30 postoperative days. Only CRP measurements in the 24 hours before or after the start of treatment are included. SI conversion factors: to convert CRP to mg/L, multiply values by 10.
ORs for the association of infection with measured C-reactive protein (CRP) levels over time in the first 30 postoperative days. Week +4 includes days 21–30 postoperative. For calculation of all ORs, the CRP stratum of 0–5.0 mg/dL was taken as a reference. Black bars denote 95% CIs (see online supplemental etable 3 in the Supplement for the exact values). CRP is reported in mg/dL, to convert to mg/L multiply values by 10.
Surgical subspecialties
Eight different surgical subspecialties were included, that is, general surgery, cardiothoracic surgery, neurosurgery, urological surgery, orthopaedic surgery, gynaecological surgery, ENT surgery and maxillofacial surgery. Most CRP measurements were performed after cardiothoracic surgery (90% of the included procedures had at least one CRP measurement in the 30-day postoperative period) and least CRP measurements after maxillofacial surgery (7% of the procedures). In all subspecialties, the association between CRP and postoperative infection was stronger in weeks 2–4 postoperatively as compared with the first week postoperatively (figure 4). The strength of the association between CRP and postoperative infection differed per surgical subspecialty. Especially in the first postoperative week, there was only a small association between CRP and infection in general, cardiothoracic surgery and orthopaedic surgery (see online supplemental etables 4–11 in the Supplement for all the subgroup analyses results).
ORs for the association of infection with measured C-reactive protein (CRP) levels over time in the first 30 postoperative days stratified per surgical subspecialty. CTS, cardiothoracic surgery; ENT, ear-nose-throat surgery; Gen, general surgery; Gyn, gynaecological surgery; Max, maxillofacial surgery; Neu, neurosurgery; Ortho, orthopaedic surgery; Uro, urological surgery. Week +4 includes days 21–30 postoperative. For calculation of all ORs, the CRP stratum of 0–5.0 mg/dL was taken as a reference (see online supplemental etables 4–11 in the Supplement for exact values). CRP is reported in mg/dL, to convert to mg/L multiply values by 10.
Differences between hospitals
Of the 170 791 CRP measurements included, 131 365 (77%) were performed in the LUMC and 39 426 (23%) in the RadboudUMC (see online supplemental etable 12 for the descriptive characteristics of the included procedures per hospital). In the LUMC, there were more patients with a CRP measurement as well as more CRP measurements per patient. This difference was largest in the first postoperative week (online supplemental etable 13 in the Supplement). The association between CRP and a postoperative infection in the LUMC and RadboudUMC was similar in both hospitals (online supplemental efigure 2 in the Supplement).
Discussion
We found that an elevated postoperative CRP was associated with postoperative infections, with a stronger association for a higher level of CRP and longer time elapsed since surgery, while in contrast, most CRP measurements were done in the first postoperative week. Hence, an imbalance seems to exist between the period in which most CRP measurements are performed and when it has the highest diagnostic value.
The stronger association between postoperative CRP and infection when more time since surgery has elapsed is in accordance with the normal early postoperative rise and fall of CRP, caused by inflammation by the surgery itself. In addition, patients who are still in the hospital more than 1 week after their surgery are more likely to have complications. Consequently, CRP measurements beyond this first week are possibly more based on clinical suspicion compared with the more routinely performed measurements in the first postoperative week. On the other hand, non-infectious postoperative complications such as fluid overload, non-septic shock, thrombosis and hypoxemia can lead to inflammation and an elevated CRP.16–19 Altogether, our results show a strong correlation between CRP and infection. In combination with clinical evaluation and additional diagnostic tests, postoperative CRP can aid in diagnosing or ruling out a postoperative infection.
Besides CRP, other biomarkers like procalcitonin have been studied for their use in the diagnosis of postoperative infections. Procalcitonin levels increase in response to bacterial infection or sepsis and are considered to be more specific for bacterial infection than CRP.11 20 However, for the purpose of diagnosing postoperative infections, procalcitonin has only been studied in small cohorts with conflicting results. In a meta-analysis in cardiac surgery patients, a mean sensitivity of 0.67 (0.47–0.82) and mean specificity of 0.73 (0.65–0.79) were found with a PPV around 50% and a NPV of >90%.21 This is similar to two other meta-analyses in gastro-intestinal and pancreatic surgery.11 20 In general, procalcitonin seems to be insufficiently specific for the diagnosis of postoperative infections, although it has a good NPV and could therefore be useful to exclude a postoperative infection when procalcitonin is low. This concurs with our results on CRP. Because we included only observational data and procalcitonin was not routinely measured, no comparison could be made between procalcitonin and CRP. Other biomarkers that have been evaluated as markers for postoperative infections include IL6, IL18, white cell count, neutrophils, lactate and surface receptor CD64.20 22–24 These studies show that none of these biomarkers was able to diagnose a postoperative infection with a high accuracy.
Between the eight different surgical subspecialties, notable differences were observed regarding the association between CRP and postoperative infection. The ORs were lowest in the first postoperative week for general surgery, cardiothoracic surgery and orthopaedic surgery (ranging between 1.0 and 5.5). Potentially, larger wound beds are created in these types of surgical interventions that in turn cause a more extensive postoperative inflammatory reaction. This contrasts with ENT, maxillofacial surgery and gynaecology, which had the highest ORs in the first postoperative week (ranging between 2.4 and 19.9).
Fewer postoperative CRP measurements per patient were performed in the RadboudUMC compared with the LUMC. Several factors could account for this difference such as variations in protocols regarding postoperative laboratory ordering, use of change in CRP instead of single CRP values in the diagnosis of infection or the use of CRP to monitor treatment. Even though the number of CRP measurements differed, the association between CRP and postoperative infections was similar, with a stronger association from the second postoperative week onwards.
This study included a high number of procedures and observational CRP measurements and comprised multiple types of surgery as well as a follow-up time of 30 days. Many previous investigations on the relationship between CRP and postoperative infections included only one surgical subspecialty, fewer procedures and had a shorter follow-up. For example, the meta-analysis of Yeung et al12 focused on colorectal surgery, included a total of 6647 patients from 23 studies and had a follow-up of 7 days postoperatively. In comparison, our study included electronic healthcare data from 42 125 procedures, providing insight into the clinical use of CRP and the value of CRP as used in clinical practice.
Limitations
Several limitations of this study need to be considered. The use of a large electronic health record database—that is, ‘big data’—made it impossible to verify every infection by manual chart review. Therefore, an action-based definition of infection was used and defined by the start of non-prophylactic antibiotics with a duration of at least 72 hours and/or an infection-related surgical re-intervention. Importantly, for a random sample of patients (n=100), manual chart review was performed and showed good correspondence between the action-based definition and diagnosis of postoperative infection by experts. It is still possible that patients without a postoperative infection have had antibiotics or a re-intervention and that this was done (partly) based on an elevated CRP. However, the Netherlands has a high standard of antibiotic stewardship, and manual labelling has other limitations, for example, interobserver variability and error rates. A second limitation is that the exact start of an infection could not be determined, but this is always difficult if not impossible. As a practical approach to this dilemma, patients were excluded from the group without a postoperative infection 1 week before the start of infection treatment to avoid CRP measurements in patients with a beginning infection being counted in the group of patients without an infection. Third, for this study, we aimed to explore the diagnostic value of CRP to assess a postoperative infection. If we would have had a more prognostic approach, differences in duration of surgery, type of anaesthesia and precautious interventions would also have been relevant to prognose a postoperative infection. But for this study, we did not adjust for these factors, also because not all possible prognostic factors have been measured. Furthermore, the lack of availability of data on subgroup membership is also the most important reason why we did not perform subgroup analyses to further explore the diagnostic relation between CRP and infection in different subgroups. Finally, we excluded patients with a preoperative CRP >2.5 mg/dL to prevent patients with a preoperative infection from being classified as having a postoperative infection. Cole et al9 previously showed that about 5% of the patients have a preoperative CRP >3.0 mg/dL which was probably related to comorbidities. Possibly, we have excluded patients with an elevated CRP due to comorbidities instead of preoperative infection. For this study, the inclusion of patients with a preoperative infection would be of greater impact than the exclusion of a patient with an elevated CRP due to comorbidities. In addition, not every patient had a preoperative CRP measured. Therefore, patients with an unknown elevated CRP could have been included in the study. Seemingly, these patients had no reason for measuring a preoperative CRP, making a preoperative infection much less likely. The study of Cole et al did not show a difference in CRP between patients with and without an infection in the first postoperative week, irrespective of the preoperative CRP, which agrees with the results of this study. Moreover, age, comorbidities and medication use could have influenced the level of CRP as well as the risk of a postoperative infection. Studies on the relation between CRP and community-acquired pneumonia and COVID-19 have shown different results for different age and comorbidity groups.25–27 Therefore, the strength of the relation between CRP and postoperative infection might differ for patients with certain comorbidities, advancing age or medication use.
Summary and conclusions
This study revealed that the association between CRP levels and postoperative infections is dependent on the CRP level, the time elapsed since surgery and the surgical subspecialty. Currently, CRP assessments are performed within the initial week after surgery, despite their limited clinical significance. Clinicians need to recognise the evolving nature of postoperative CRP values for the diagnosis of postoperative infections and advance to more selective and consciously performed CRP assessments to optimally use its diagnostic capacities. Moreover, these results elucidate the difficulty of using CRP in clinical prediction models and are therefore highly significant for the development of new clinical prediction models incorporating CRP.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
References
Footnotes
Collaborators The PERISCOPE study group: all above mentioned authors, Jogchum J Beltman, MD, PhD (LUMC); Bas Bredie, MD, PhD (RadboudUMC); Jaap F Hamming, MD, PhD (LUMC); Pieter de Heer, PhD (Rigshospitalet); Merlijn Hutteman, MD, PhD (RadboudUMC); Maxime T M Kummeling, MD, PhD (LUMC); Rolv-Ole Lindsetmo, MD, PhD (University Hospital of North Norway); Wilco C Peul, MD, PhD (LUMC); Karin Ellen Veldkamp, MD, PhD (LUMC).
Contributors AMvB: methodology, analysis, writing original draft. SLvdM: methodology, analysis, data collection, writing-review and editing. BFG: data collection, writing-review and editing, conceptualisation. HvG: writing-review and editing, conceptualisation. NvG: methodology, writing-review and editing. MSA: writing-review and editing, conceptualisation, supervision. MGJvB: writing-review and editing, conceptualisation, supervision, guarantor. The periscope study group: data collection, conceptualisation, writing-review and editing.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests BG is currently CEO and majority shareholder of Healthplus.ai B.V. and subsidiaries. SvdM works as a data scientist and PhD at Healthplus.ai B.V. and LUMC. SvdM owns share options in Healthplus.ai B.V. AvB, HvG, NvG, SA, MdB and the other members of the PERISCOPE study group have no competing interests to declare.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.