Article Text
Abstract
Introduction While recent research suggests that acupuncture may offer benefits to individuals with diarrhoea-predominant irritable bowel syndrome (IBS-D), high-quality studies are scarce in this area. We intend to investigate the efficacy and safety of individualised sensitised acupuncture in IBS-D.
Methods and analysis The study is designed as a large-scale, multicentre, two-arm, randomised clinical trial involving 326 patients diagnosed with IBS-D. Participants will be randomly allocated into the acupuncture or the sham acupuncture group in a 1:1 ratio. Both groups will undergo 15 sessions over 6 weeks. The primary outcome is the effective response rate at week 6, with secondary outcomes including the effective response rate at alternative time points, percentage of patients with 3 or more effective response weeks throughout the treatment duration, IBS Symptom Severity Scale, IBS-Quality of Life, Patient Health Questionnaire-9, Adequate Relief of IBS Symptoms Scale, Extraintestinal Symptoms Scale and other symptoms.
Ethics and dissemination The study protocol has been approved by the Medical Ethics Committee of Beijing University of Chinese Medicine (project number: 2023BZYLL0102) and the ethics committees of other participating institutions. Each participant will be required to provide written consent before enrolment. The study results will be submitted for publication in a peer-reviewed journal.
Trial registration number ChiCTR2300078321.
- Acupuncture
- Irritable Bowel Syndrome
- Randomized Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is a large-sample clinical trial designed to evaluate the efficacy and safety of acupuncture at sensitive acupoints in patients with diarrhoea-predominant irritable bowel syndrome (IBS-D).
The treatment programme involves a personalised selection of sensitive acupoints based on individual patients’ sensitivity levels.
Methodological rigour will be applied, including adequate randomisation, objective measures, rigorous training of trial personnel and clear role separation.
The absence of blinding of acupuncturists could introduce bias and compromise the validity and reliability of the results.
Eligible participants will be recruited from five hospitals throughout China, and the findings might have certain limitations when extended to patients with IBS-D in other nations.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterised by recurrent abdominal pain with or without abnormal bowel habits.1 The global prevalence of IBS is estimated to be as high as 11.2%, with a higher prevalence among female individuals and a lower incidence among individuals aged 50 years or older.2 3 According to predominant faecal characteristics, IBS can be categorised into four subtypes: IBS with predominant constipation (IBS-C), IBS with predominant diarrhoea (IBS-D), IBS with mixed symptoms (IBS-M) and IBS unclassified (IBS-U). IBS-D is the most prevalent, accounting for three-quarters of diagnosed cases.4–6 Although IBS is not life-threatening, it significantly impacts patients’ quality of life and imposes a substantial economic and medical burden on society, with costs in the USA reaching US$10 billion.7
The primary treatment for IBS-D involves pharmacotherapy, such as using antidiarrhoeal agents, antispasmodics and antidepressants, as first-line or second-line therapies. However, most medications usually provide only temporary relief of specific symptoms and are associated with a high recurrence rate.8 An escalating number of patients with IBS are seeking out complementary and alternative therapies, and acupuncture is being acknowledged as a pivotal alternative therapy for IBS. A meta-analysis9 of IBS with 27 randomised controlled trials (RCTs) reveals that acupuncture showed notable effectiveness in alleviating symptoms, superior to certain symptom medications. Recent high-quality RCTs have further substantiated this perspective. Collectively, acupuncture has the potential to alleviate the symptoms and enhance the quality of life of patients with IBS compared with both pharmacological interventions and sham acupuncture.10 Our previous study showed clinically meaningful improvement in IBS-D symptoms. The effective response rate of patients in both the specific acupoints and the non-specific acupoints group was 46.7%, yet there was no significant difference in the effective response rates when compared with the sham acupuncture group.11 However, the majority of current studies on acupuncture for IBS-D still yield inconsistent conclusions and lack sufficient methodology.12–15 MacPherson et al12 randomised 233 participants with IBS to receive either a short course of traditional acupuncture combined with usual care or usual care alone, with equal distribution between the two groups. The study found that, at 3 months, the IBS Symptom Severity (IBS-SSS) scores of participants receiving acupuncture treatment significantly decreased. However, the study has some limitations, including the lack of blinding of participants and the use of the relatively subjective IBS-SSS score as the primary outcome measure. Lembo et al14 compared the efficacy of acupuncture and sham acupuncture treatments over six sessions across 3 weeks in patients with IBS, and the results indicated no significant differences in symptom improvement between the acupuncture and the sham acupuncture group at the end of the treatment. However, compared with the waitlist control group, both groups showed significant improvements. Although this study included a sham acupuncture control group, it still has limitations, such as a relatively short treatment duration and the use of a subjective primary outcome. At the same time, we observed that in some clinical studies in which the efficacy of acupuncture for IBS was not found, most used standardised acupoint selection programmes without considering individual patient variation.15–17 In clinical therapy, acupuncturists typically conduct examinations of acupoints on the patient’s body surface before treatment and select sensitive acupoints for acupuncture. Although these patients share the same disease, the acupoint programme may be various. Thus, we contend that personalised acupuncture therapy could elevate the efficacy of acupuncture in patients afflicted with IBS.
The precise selection of acupoints is crucial in determining the therapeutic efficacy of acupuncture. Historically, acupuncturists targeted highly sensitive acupoints to achieve optimal clinical effectiveness. The relationship between disease condition and acupoints is significant, as lesions in internal viscera or deep tissues can cause changes in specific acupoints related to the affected organs.18 In the diseased state, specific acupoints will demonstrate sensitive conditions. The sensitivity of acupoints is mainly manifested by local skin swelling, heat and pain, among which the decrease in the pain threshold is the most common.19 The sensitive acupoints are linked to the clinical effectiveness of acupuncture. The efficacy of treating sensitive acupoints has recently been substantiated by studies encompassing chronic neck pain, bronchial asthma, stable angina pectoris, knee osteoarthritis and various other conditions.20–24 Studies have also shown that patients with gastrointestinal disorders often have sensitive acupoints, particularly those that are sensitive to pain.22 Pain-sensitive points are regions that react in response to stimulation with a minimum force applied on the skin by external pressure and are characterised by a decrease in the pain threshold.25
Based on this, we designed a multicentre randomised controlled clinical trial. This study implements personalised sensitive acupoint acupuncture treatment for participants with IBS-D. To adapt to the chronic and functional nature of IBS, this study was designed with an appropriate treatment duration and number of sessions. The primary outcome for evaluating treatment efficacy is the effective response rate, combining both objective and subjective outcome measures. This study aims to investigate the efficacy of personalised sensitive acupoint acupuncture over 6 weeks (15 sessions) in alleviating abdominal pain and reducing the number of days with loose stools in patients with IBS-D. This study also aims to investigate the efficacy and safety of sensitive acupoint acupuncture in treating IBS-D in order to provide high-quality clinical evidence for acupuncture treatment of IBS-D.
Methods and analysis
Study design
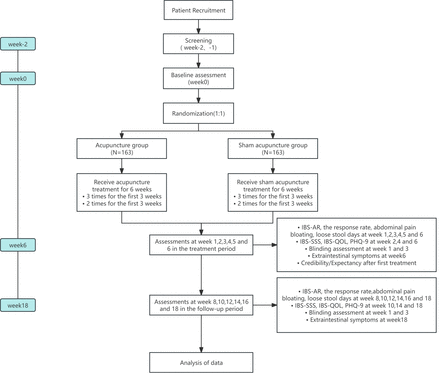
This study is a large-scale, multicentre, two-arm, randomised clinical trial designed to test the efficacy of sensitive acupoint acupuncture treatment for patients with IBS-D. It will test the following hypothesis: 6 weeks of sensitive acupoint acupuncture treatment will result in a better effective response in patients with IBS-D compared with sham acupuncture. The trial will be conducted at seven institutions in China: (1)Beijing Fangshan District Liangxiang Hospital, Beijing, (2) Hospital of Chengdu University of Traditional Chinese Medicine, (3) Affiliated Hospital of Shandong University of Traditional Chinese Medicine, (4) Hospital of Jiangxi University of Traditional Chinese Medicine, (5) Hebei Provincial Hospital of Traditional Chinese Medicine, (6) Yunnan Provincial Hospital of Traditional Chinese Medicine and (7) The Second Affiliated Hospital of Fujian University of Traditional Chinese Medicine. Eligible patients with IBS-D will be randomly assigned to either the acupuncture group or the sham acupuncture group in a 1:1 ratio. The trial will consist of three phases: a 2-week screening phase (weeks -2 and -1), a 6-week treatment phase (weeks 1–6) and a 12-week follow-up phase (weeks 7–18), totalling 20 weeks. The flow chart of this study is shown in figure 1, and the assessment time point is shown in online supplemental table 1. The study protocol was approved by the Medical Ethics Committee of the Beijing University of Chinese Medicine and the ethics committee of each research centre before study commencement. All study personnel will receive unified training on the implementation of the test. The recruitment commenced on 5 December 2023 and is expected to continue until December 2025.
Supplemental material
Trial flow chart. IBS, irritable bowel syndrome; IBS-AR, Adequate Relief of IBS Symptoms Scale; IBS-QOL, IBS-Quality of Life; IBS-SSS, IBS Symptom Severity Scale; PHQ-9, Patient Health Questionnaire-9.
Participants
The subjects will be recruited through the outpatient clinic of the research centre, recruitment advertisements and WeChat platform. Participant data will be collected by the REDCap electronic data capture (EDC) system. Participants will be required to maintain a defecation diary throughout a 2-week screening period prior to enrolment. Each participant will sign an informed consent form before randomisation.
Inclusion criteria
Aged between 18 and 75 years old, both male and female.
Fulfilled the Rome IV diagnostic criteria for IBS-D.
The daily defecation diary from the past 2 weeks indicates the presence of Bristol stool patterns of type 6 or 7 for a minimum duration of 4 days, while type 1 or 2 only occurred for a maximum duration of 4 days; furthermore, the average daily abdominal pain score was equal to or greater than 3 during the previous week.
No acupuncture treatment in the last 6 months.
Exclusion criteria
Other subtypes of IBS: IBS-C, IBS-U or IBS-M.
Inflammatory bowel disease, microscopic colitis, history of coeliac disease, Crohn’s disease and other organic bowel diseases. Normal endoscopy results within 2 years are required for those age 50 or older or with the following alarming signs: unexplained weight loss (weight loss equal to or greater than 10% within 3 months), haematochezia other than from haemorrhoids or anal fissures, nocturnal diarrhoea or family history of colorectal cancer.
Taking antidepressants and medications with therapeutic effects on symptoms of IBS within 2 weeks before treatment, including traditional Chinese medicine or Chinese patent medicine, antidiarrhoeal agents, antispasmodic agents, intestinal antibiotics, probiotics, etc.
Diabetes, thyroid dysfunction, severe acute/chronic organic disease and kidney or liver disease.
History of abdominal surgery, excluding appendectomy, haemorrhoidectomy or polypectomy, performed more than 3 months ago.
Currently in the period of pregnancy or lactation.
History of substance abuse, including alcohol and drug use.
Enrolled in another clinical trials.
The exclusion criteria will apply to individuals who meet one or more of the aforementioned requirements.
Randomisation and blinding
In this study, eligible patients with IBS-D will be randomly allocated to either the acupuncture group or the sham acupuncture group in a 1:1 ratio using the REDCap EDC system. Randomisation will be stratified by recruitment site, with a dynamic block size of 4, 6 or 8. The randomisation sequences will be generated by an independent statistician and securely stored within the REDCap EDC system. Before the first acupuncture treatment, the acupuncturist will receive the assigned grouping information through the REDCap EDC system. Different roles within this study will have varying levels of access and permissions to the REDCap EDC system. Only the acupuncturist and their assistant will have access to the participant’s allocation information. Participants, recruiters, outcome evaluators, data managers and statistical analysts will be blinded to the participant’s randomisation group. A sham acupuncture group will be established in this study. Patients will receive treatment in individual cubicles to ensure blinding and are strictly prohibited from engaging in any form of communication during the treatment sessions. A blinding assessment will be performed on participants at the early and midtreatment stages.
Interventions
The acupuncture group and the sham acupuncture group will be set in this study. Interventions will be conducted by licensed acupuncturists with a minimum of 3 years of clinical experience. Participants will undergo a 6-week acupuncture treatment, with sessions scheduled three times per week for the initial 3 weeks and two times per week for the subsequent 3 weeks, totalling 15 sessions lasting 30 min each. Before study commencement, personnel in different roles will receive specialised training, and only those who have completed the training will be eligible to participate.
During treatment, loperamide (Xi’an Janssen Pharmaceutical) will be provided to the participants as a contingency medication, administered by a gastroenterologist only in cases of severe and intolerable symptoms such as acute abdominal pain. The medications used will be carefully documented throughout the trial, including any non-IBS-related medications.
The participant will be discontinued from the study if any of the following circumstances arise: experiences a serious adverse event, experiences other conditions that impede the outcomes assessment, has poor compliance or expresses unwillingness to continue during the course of the clinical trial. Poor compliance is defined as the use of non-protocol treatments or medications, exceeding the prescribed dosage of rescue medication or repeatedly missing treatment sessions, which constitutes a serious deviation from the study protocol.
Acupuncture group
In the acupuncture group, acupuncture will be administered at six high-sensitive acupoints. The acupuncture sites and the hands of the acupuncturist must undergo strict sterilisation with 75% alcohol. Acupuncture treatment will use disposable sterile acupuncture needles (0.30×40 mm; Suzhou Huatuo Medical Equipment). The manipulation will be performed to achieve deqi, with the degree of local acid distension serving as the indicator of successful treatment.
Selection and localisation of acupoints repository
After a comprehensive literature review and clinical experience, we have identified 17 acupoints that are frequently used in treating IBS-D (figure 2 and online supplemental table 2).26–28 The acupoint locations will be determined according to the national standard of the People’s Republic of China (GB/T12346-2021)including the name and location of acupoints
Location of acupoints and non-acupuncture points. Red dots: location of acupoints in the sensitive acupoints group; blue dots: location of non-acupuncture points in the sham acupuncture group. The figure has been modified based on www.3Dbody.com. (A) Ventral view, (B) dorsal view, (C) view of the upper extremity, (D) anterior aspect view of the lower extremity, (E) lateral view of the lower extremity and (F) medial aspect view of the lower extremity.
Assessment of pain threshold at acupoints
The pain threshold will be measured twice at each of the 17 acupoints in a predetermined sequence (upper limbs→abdomen→lower limbs→back) using a Wagner Digital Force Gauge (FDX 50; Wagner, Greenwich, Connecticut, USA). If the difference between the two measurements is more than 5 N, a third measurement will be performed and the pain threshold measured will be averaged.
Identification of intervention acupoints
The acupoints on the abdominal midline, including zhongwan (CV12), xiawan (CV12), qihai (CV6) and guanyuan (CV4), will be assessed for pain threshold, with selection of the first two acupoints based on their ranking from low to high. Bilateral acupoints will be selected based on the ratio of bilateral pain threshold (right/left), with the absolute value of ‘ratio-1’ being sorted in a descending order, and the top four acupoints will be chosen.
During the treatment period, the acupoint pain thresholds will be measured once every 3 weeks during the treatment, specifically before the first treatment in the first week and before the first treatment in the fourth week. Acupoints will be adjusted according to the results.
Sham acupuncture group
Five non-acupoints will be selected (online supplemental table 3). The acupuncture sites and the hands of the acupuncturist must undergo strict sterilisation with 75% alcohol. Acupuncture treatment will use disposable sterile acupuncture needles. Shallow needling method will be used to penetrate the skin of the participants. The needle tip will only penetrate 2–3 mm under the skin, without manipulation and without reaching deqi. The pain thresholds of the five non-acupoints will also be measured and recorded before the first treatment in the first week and the first treatment in the fourth week.
Outcomes
Primary outcome
The primary outcome will be the effective response rate at week 6. The effective response rate is defined as the percentage of patients with a mean improvement of 30% from baseline in the worst abdominal pain and a 50% reduction from baseline in the number of days with loose stools. These criteria align with the recommended outcomes by the Food and Drug Administration (FDA).29
The defecation diary will be used to record participants’ defecation and abdominal pain, bloating and other symptoms. The worst abdominal pain score in the past 24 hours will be assessed using a numerical rating scale (on a scale from 0 to 10, with 0 indicating no pain at all and 10 indicating the worst pain the patient could imagine) and the weekly average will be calculated. Episodes of loose stool will be defined as the presence of at least one stool type 6 or 7 on a given day.
Secondary outcomes
The secondary outcomes will include the effective response rate at other time points and the percentage of patients experiencing 3 or more effective response weeks during treatment. The IBS-SSS, IBS-Quality of Life (IBS-QOL), Patient Health Questionnaire-9 (PHQ-9), Adequate Relief of IBS Symptoms Scale (IBS-AR), Extraintestinal Symptoms Scale, abdominal pain symptoms, bloating, loose stool days, blinding assessment and the Credibility/Expectancy Questionnaire will also be evaluated as secondary outcomes.
IBS-Symptom Severity Scale
The IBS-SSS30 is a comprehensive assessment scale that evaluates the overall symptoms of IBS, encompassing clinical manifestations and their correlation with patients’ quality of life. The scale evaluation includes five domains: the severity of abdominal pain, the duration of abdominal pain, the severity of abdominal distension, satisfaction with defecation and impact on quality of life. Each domain is scored on a scale from 0 to 100, with the total score ranging from 0 to 500 and with higher scores indicating worse symptoms. The evaluation time points will be at baseline and at 2, 4, 6, 10, 14 and 18 weeks after the start of treatment.
IBS-Quality of Life
The IBS-QOL31 scale comprises 34 items and is organised into eight dimensions: dysphoria, health worry, body image, interference with activity, food avoidance, social reaction, sexual and relationship. The scores range from 0 to 100, with higher total scores indicating higher quality of life. The evaluation time points will be at baseline and at 2, 4, 6, 10, 14 and 18 weeks after the start of treatment.
Patient Health Questionnaire-9
The PHQ-932 is a diagnostic instrument used to assess common mental disorders, with scores ranging from 0 to 27. A higher total score indicates a more severe psychological condition. The evaluation time points will be at baseline and at 2, 4, 6, 10, 14 and 18 weeks after the start of treatment.
Adequate Relief of IBS Symptoms Scale
The IBS-AR33 scale includes a dichotomous item that asks patients ‘Have your IBS symptoms been adequately relieved during the past week?’ This scale will be employed to evaluate symptom relief in individuals with IBS. The evaluation time points will be at 1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16 and 18 weeks after the start of treatment.
Extraintestinal Symptoms Scale
The Extraintestinal Symptoms Scale34 is a rating scale for 15 prevalent symptoms frequently reported as adverse events in clinical drug trials. The 15 symptoms include bad dreams, excessive sleepiness, insomnia, fatigue, inability to concentrate, irritability, dry mouth, headache, weakness, dizziness, joint pain, muscle pain, nasal congestion, skin rash and bruising. Each symptom is scored on a scale of 0–5, with 0 indicating absence and 5 indicating severity. The severity scores for the 15 symptoms are summed to calculate a composite score for each participant, resulting in an overall symptom burden score ranging from 0 to 75. The evaluation time points will be at baseline and at 6 and 18 weeks after the start of treatment.
Blinding assessment and Credibility/Expectancy Questionnaire
All participants will be asked the question ‘Do you believe you have received traditional acupuncture or modern acupuncture?’ for blind assessment during week 1 and week 3. Following the initial treatment, participants will be mandated to complete the Credibility/Expectancy Questionnaire35 to evaluate their perceived credibility of the treatment and their expectations. The Credibility/Expectancy Questionnaire consists of two components: the credibility of the treatment and the patient’s expectations of the treatment. The aim is to assess whether patients’ expectations influence the actual effectiveness of the treatment and whether their confidence in the treatment predicts treatment adherence and outcomes.
Adverse events
Adverse events, both related and unrelated to acupuncture, will be promptly documented in the REDCap EDC system during both the treatment and follow-up periods. All adverse events will be managed symptomatically by acupuncturists and doctors with relevant specialties. The assessment of the incidence and severity of adverse events will be conducted after the treatment period (week 6) and at the end of the follow-up period (week 18).
Data management
Both paper files and electronic documents will be preserved for at least 5 years after publication. Original data can be accessed by contacting the corresponding author. Patient information will remain anonymous, including name, ID number and mobile phone number. An electronic case report form (eCRF) will be used for data collection during the trial. Paper defecation diary cards will be provided to participants who do not have easy access to electronic devices. Each centre will implement a standardised REDCap EDC system and uniformly printed paper test materials. Independent monitors will monitor the eCRF data in real time to guarantee the completeness and promptness of data collection, without interfering in the trial. Any changes to the data will be traceable through the eCRF.
Quality assurance
Before the initiation of the study, all trial personnel underwent comprehensive training on the study objectives, patient screening, intervention modalities and evaluation follow-up, as well as familiarisation with the operation of the REDCap EDC system. The trial personnel will maintain all paper documents, such as defecation diary cards and acupuncture record forms. Access permissions for the REDCap EDC system will vary depending on the roles within the study. A team of monitors will be established to monitor the trial data both online and offline. We will conduct one fixed online monitoring session per month, with untimed checks on the completeness and timeliness of data uploads. When 10% and 90% of participants have been recruited, the monitoring team will carry out fixed offline monitoring. Additionally, if any centre encounters irregularities in trial implementation or experiences slow recruitment progress, on-site monitoring will be conducted for that centre. The monitoring will encompass all trial procedures, including patient recruitment, intervention implementation, assessment and data input. The contact information and the residential address of the trial participants will be recorded, and regular communication will be maintained to enhance participant adherence to the protocol. When participants miss multiple appointments, they will be contacted to determine the reasons for their absences.
Sample size
According to the previous research of our group (unpublished. Prof CZ Liu. 2021-2022), the effective response rate will be estimated at 55.9% in the acupuncture group and 38.8% in the sham acupuncture group. We used a two-sided test with α=0.05 and β=0.2. The sample size calculation indicated that each group should have a minimum of 130 effective cases. Considering a dropout rate of 20%, a total of 163 cases will be recruited for each group, resulting in a total of 326 cases in both groups.
Statistical analysis
The study will use SAS V.9.3 software for data analysis. Statistical analysis will be performed by an independent statistician who is blinded to group assignments. Continuous data will be presented as either mean±SD or median and IQR, while categorical data will be reported as frequencies (percentages). A two-sided test level of 0.05, with a p value less than 0.05, will be considered to indicate statistical significance.
All analyses will adhere to the intention-to-treat protocol, which includes all randomised patients with available baseline information. Missing data will be imputed using multiple imputations. A per-protocol population analysis of the primary outcome measure will be conducted for participants who completed no less than 80% of the treatment. Safety analyses will be based on participants who will receive at least one session of treatment.
For the primary outcome, a logistic generalised linear mixed model will be used to test for differences between groups. The secondary outcomes will be compared between groups using t-test or Wilcoxon rank-sum for continuous data and χ2 test or Fisher’s exact test for categorical data.
Patient and public involvement
None.
Ethics and dissemination
The protocol has obtained approval from the Medical Ethics Committee of Beijing University of Chinese Medicine (project number: 2023BZYLL0102) and the medical ethics committees of five hospitals. This study has been registered on the Chinese Clinical Trial Registry (ChiCTR) platform (number: ChiCTR2300078321). A written informed consent (online supplemental file) will be obtained from each participant before any study procedure is performed, according to good clinical practice. The findings of this study will be submitted for publication in a peer-reviewed journal.
Supplemental material
Trial status
This trial is currently recruiting participants. The recruitment commenced on 5 December 2023 and is expected to continue until December 2025.
Discussion
IBS-D is the most prevalent subtype of IBS, characterised by chronic, recurring episodes and comorbid mental health conditions such as anxiety and depression, which significantly impact patients’ quality of life.36 A large-scale, multicentre, two-arm, randomised clinical trial is designed to assess the efficacy of acupuncture at sensitive acupoints for IBS-D.
The selection of acupoints plays a crucial role in acupuncture treatment. This study will implement a personalised programme for acupoint selection based on the level of acupoint sensitisation. In the ancient Chinese medical book, Medical Classic of the Yellow Emperor, it is documented that excellent therapeutic efficacy can be obtained by choosing the acupoints with sensitive manifestations, such as induration, nodular and tenderness, in the context of acupuncture treatment. In a randomised neuroimaging trial, 99 patients with chronic neck pain were randomised to either true acupuncture or sham acupuncture. The true acupuncture group and the sham acupuncture group were treated with standard acupuncture treatment at the pain-sensitive acupoints and shallow acupuncture at non-acupoints, respectively. Both groups received treatment for 4 weeks. After treatment, sensitive acupoint acupuncture showed a significant improvement in both the severity and duration of pain when compared with the sham acupuncture group.37 IBS-D is characterised by a range of symptoms, which can vary between individual patients. Therefore, a personalised acupuncture regimen may yield greater efficacy in alleviating IBS symptoms among participants. A study randomly dichotomised 80 patients with IBS-D into a control group and a treatment group. The control group received treatment with pinaverium bromide, while the treatment group underwent therapy with pinaverium bromide in combination with heat-sensitive moxibustion (a novel form of moxibustion that identifies thermally sensitive acupoints through suspended moxibustion).38 The results indicated that the treatment group exhibited a significant improvement in IBS-SSS and quality of life when compared with the control group. Lei et al39 identified that the sensitive acupoint of IBS-D model rats was the dachangshu (BL25). Subsequently, the rats were stratified into sensitive and non-sensitive acupoint groups, revealing a significantly enhanced efficacy of electroacupuncture in the sensitive acupoint group compared with the non-sensitive counterpart. In this study, pain sensitisation will be used as the criterion for acupoint selection due to its prevalence as a manifestation of sensitisation.23 The degree of change in pain threshold will objectively reflect the intensity of acupoint sensitisation and may be correlated with the disease state.40 Additionally, a study has shown that individuals with IBS have a lower acupoint pain threshold compared with healthy individuals.41 However, current research on the efficacy of sensitive acupoints in patients with IBS is predominantly theoretical, with a notable absence of high-quality clinical studies.
The reporting of this multicentre RCT will adhere to the guidelines of the Consolidated Standards of Reporting Trials42 and the STandards for Reporting Interventions in Clinical Trials of Acupuncture.43 One major challenge in clinical acupuncture research is establishing an appropriate sham acupuncture control. Common types of sham acupuncture used in clinical trials include needling insertion at acupuncture points, needling insertion at non-acupuncture points, non-insertion at acupuncture points and non-insertion at non-acupuncture points.44 Maintaining blinding in Chinese participants, many of whom have acupuncture experience, is particularly difficult. To address this, the sham acupuncture group will receive shallow needling at non-acupoints, with pain threshold measurements taken. Participants will be separated during treatment to ensure blinding and compliance. The FDA-recommended effective response rate will serve as the primary outcome, offering a more rigorous assessment than the IBS-SSS used in previous studies. Randomisation will be conducted using the REDCap EDC system, with computer-generated sequences and restricted access to allocation information to prevent bias. Additionally, all trial personnel will undergo professional training before the trial commences, with independent supervisors overseeing data monitoring and trial implementation to uphold trial integrity.
This study has several limitations. First, due to the distinctive characteristics of acupuncture intervention, implementing blinding of acupuncturists may not be feasible. Second, time and resource constraints will only allow for pain threshold measurements to be taken twice for each acupuncturist. Subsequently, patients will receive treatment at the same acupoints for 3 weeks based on the measured data. The stability of higher sensitised acupoints over 3 weeks cannot be guaranteed. Finally, the generalisability of our findings to patients with IBS-D outside of China may be limited.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors Submitting author: Z-TF. Corresponding author: N-NY. Collaborators (group authorship): C-ZL, QZ, L-LC, X-BH, J-HG, Y-WX, YW, J-WY, HZ, Y-DL. C-ZL and N-NY proposed and initiated the study. ZF, N-NY, C-ZL, J-WY and YW participated in designing, drafting and revising the manuscript. QZ, L-LC, X-BH, J-HG and Y-WX coordinated the study. C-ZL, QZ, L-LC, X-BH, J-HG and Y-WX sought ethical approval. J-WY, YW, HZ and Y-DL assisted in manuscript revision. N-NY is the guarantor.
Funding The study is funded by the National Key Research and Development Program for the Modernization of Traditional Chinese Medicine (no: 2022YFC3500605).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.