Article Text
Abstract
Introduction Antipsychotics are likely to cause weight gain owing to increased appetite and other metabolic disturbances in patients with schizophrenia on prolonged medication. Conventional high-frequency repetitive transcranial magnetic stimulation has been employed to treat people with obesity and has shown certain effectiveness. The goal of this clinical trial is to evaluate the efficacy of intermittent theta burst stimulation (iTBS) in ameliorating appetite increase and weight gain induced by antipsychotics in patients with schizophrenia.
Methods and analysis In this randomised, double-blind, sham-controlled trial, 60 participants will be enrolled and allocated (1:1) to receive active or sham iTBS on the dorsolateral prefrontal cortex for 5 consecutive days. Appetite, body mass index, clinical symptoms, cognitive function and laboratory indicators will be assessed at baseline, after 5 days of treatments, and at 2 weeks and 4 weeks after all treatments. MRI examination will be conducted to detect brain structure, perfusion and functional connectivity. Data analysis will be conducted in a modified intention-to-treat population. The results of the study will provide evidence on the effectiveness and feasibility of iTBS in improving increased appetite induced by antipsychotics and explore the underlying neuroendocrine pathway affected by the intervention. The primary objective is to evaluate the efficacy of iTBS in weight gain in patients with schizophrenia taking antipsychotics. The secondary objective is to identify the neuroendocrine changes related to appetite in response to iTBS by assessing the variables of cognitive control, glucolipid metabolism and brain activity.
Ethics and dissemination The study protocol has been approved by the National Clinical Medical Research Center Ethics Committee of The Second People’s Hospital of Dali Bai Autonomous Prefecture (no: 2023YN3) and The Second Xiangya Hospital (no: 2024K008). Written informed consent will be obtained voluntarily before enrolment. The results will be disseminated through publication in peer-reviewed journals and presentation at international conferences.
Trial registration number NCT05783063.
- Transcranial Magnetic Stimulation
- Magnetic resonance imaging
- Schizophrenia & psychotic disorders
- Obesity
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Transcranial Magnetic Stimulation
- Magnetic resonance imaging
- Schizophrenia & psychotic disorders
- Obesity
STRENGTHS AND LIMITATIONS OF THIS STUDY
The primary outcome measurement is objective and easy to track.
By integrating multiomics analyses, this study employs a systems biology approach to explore the interplay between gut microbiota, metabolism and neuroimaging markers.
The study framework will enhance the understanding of neuroendocrine mechanisms underlying appetite regulation in antipsychotic-treated patients, offering insights for personalised and targeted interventions.
The small sample size limits the power to fully assess efficacy.
Introduction
Studies have shown that 58.6% of patients with schizophrenia experience weight gain or obesity, which is associated with a twofold to threefold increased risk of mortality from cardiovascular disease or diabetes compared with healthy individuals.1 2 Evidence-based studies indicate that antipsychotics, including olanzapine and risperidone, can induce metabolic abnormalities such as weight gain, hyperglycaemia, insulin resistance and hyperlipidaemia.3 These metabolic disturbances can reduce patient adherence to prescribed treatment, disrupt therapeutic protocols and increase the risk of disease recurrence.4 5 Notably, greater weight gain during the first year of antipsychotic treatment has been observed, highlighting the importance of prompt metabolic monitoring and management.6 Therefore, early intervention to manage the increased appetite associated with antipsychotics is crucial in ensuring the effectiveness of pharmacological therapy and minimising side effects.
Although the exact mechanism by which antipsychotics contribute to obesity remains unclear, existing evidence suggests that increased appetite plays a vital role.7 8 Lungu et al9 have reported that both antipsychotics and disease severity may interfere with appetite regulation. The dosage of antipsychotics is associated with both the presence and intensity of food cravings, influencing the desire and urge to eat. Impaired cognitive control is a significant factor contributing to increased appetite induced by antipsychotics in patients with schizophrenia. Additionally, cognitive inhibition of eating is negatively correlated with fluctuations in cholesterol levels in patients with schizophrenia after taking olanzapine.10 Stauffer et al11 have found that reduced cognitive control, increased hunger and binge eating are significant predictors of weight gain of ≥1 kg. In patients undergoing long-term antipsychotic treatment, increased sensitivity to food-related cues has been observed in several brain regions, including the frontal cortex, fusiform gyrus, amygdala and insula.9 10 12 13 The dorsolateral prefrontal cortex (DLPFC) is believed to play a key role in the cognitive regulation of eating behaviour, possibly by integrating reward-related cues and exerting top-down control over dopamine release in the reward pathway, which includes the nucleus accumbens and the ventral tegmental area.14
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique capable of modulating brain function, neurotransmitter activity, receptor expression and neuroplasticity15 by regular, rhythmic, repetitive and high-frequency (>5 Hz) stimulation.16 High-frequency rTMS (HF-rTMS) targeting the DLPFC could enhance both proactive and reactive cognitive control in healthy individuals.17 18 Several randomised controlled trials (RCTs) exploring the effectiveness of HF-rTMS in obesity and eating desire have chosen the left or bilateral DLPFC as stimulation targets, typically delivering 10 Hz stimulation twice weekly for 2–4 weeks.19 20 Additionally, Ferrulli et al and Luzi et al21 22 have applied 18 Hz deep HF-rTMS to bilateral DLPFC and insula in obese patients three times per week for 5 weeks. Their findings demonstrated considerable weight loss associated with reduced appetite, which could last for up to 1 year. Moreover, an exploratory study has reported the therapeutic efficacy of 10 Hz rTMS targeting DLPFC in obesity in patients with chronic schizophrenia.23 Meanwhile, HF-rTMS overleft DLPFC has also revealed an intermediate effect size in alleviating negative symptoms in schizophrenia.24 Theta burst stimulation (TBS) is a new rTMS paradigm characterised by triplet 50 Hz pulses, repeated at 5 Hz. TBS includes intermittent theta burst stimulation (iTBS), which promotes long-term potentiation (LTP); and continuous theta burst stimulation (cTBS), which induces long-term depression.25 The TBS mode significantly reduces the stimulation duration while employing identical pulse dosages. Kang et al26 have demonstrated that applying cTBS to the left primary motor cortex (M1) over 25 sessions effectively inhibited olanzapine-induced weight gain in drug-naïve patients with first-episode schizophrenia by enhancing cognitive restraint over food intake. They further provided evidence that 50 accelerated cTBS sessions over M1 led to a significant reduction in body weight and body mass index (BMI) by altering motivated attention and emotional processing in patients with comorbid obesity and schizophrenia.27 Nonetheless, iTBS, despite its excitatory effects comparable to HF-rTMS, has not been explored as a treatment for obesity in patients with schizophrenia. Given the therapeutic benefits that can be achieved within a relatively short period after 1 week of treatment, iTBS may offer a promising, fast, low-cost and effective strategy for managing antipsychotic-induced appetite increase and metabolic disturbances.
Based on the above-mentioned research, appetite increase and weight gain induced by antipsychotics in schizophrenia may be associated with impaired cognitive control and dysregulated reward processing in DLPFC. Applying iTBS to DLPFC may reduce appetite and improve metabolic disturbances in this population. Therefore, we designed an RCT to explore the efficacy, safety and tolerability of iTBS, as well as its potential neural network, in mitigating increased appetite and weight gain induced by antipsychotics in patients with schizophrenia.
Aims and hypotheses
Our primary aim is to determine whether a 5-day course of iTBS over the DLPFC can reduce weight gain, as measured by BMI, at 4-week follow-up after completing the intervention in patients with schizophrenia taking antipsychotics. Our primary hypothesis is that participants who receive iTBS will have a lower BMI compared with the control group at 4-week follow-up.
Our secondary aim is to identify the neuroendocrine changes related to appetite regulation in response to iTBS by assessing the variables involving appetite, cognitive function, glucolipid metabolism and brain activity. Our main secondary hypothesis is that iTBS can improve growing appetite, glucolipid indicators and gut bacteria dysregulation. Additionally, general cognitive function, cognitive control indexed by reaction time and accuracy in stop signal task (SST), and impulsiveness indexed by discounting rate in delay discounting task (DDT) will be improved. Moreover, metabolism-related functional and structural brain alterations detected by MRI will show signs of restoration in patients with schizophrenia. Finally, adverse events will be monitored to evaluate the safety of the intervention.
Methods
Study design and objectives
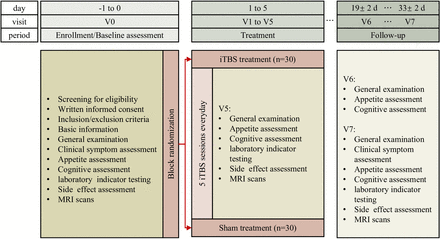
The study is designed as a randomised, double-blind, sham-controlled clinical trial. After eligibility screening, participants will be randomly assigned to the active group or the sham control group in a 1:1 ratio. Each patient will receive a total of 25 iTBS or sham stimulation sessions over a consecutive 5-day period, in addition to their optimal pharmacotherapy as prescribed by licensed psychiatrists. Outcome assessments will be conducted before, during and after the intervention. The follow-up evaluations will be scheduled at 2 weeks and 4 weeks after the completion of the intervention (figure 1).
Study flow diagram. iTBS, intermittent theta burst stimulation; V0–V7: study visits 0–7.
Sampling
Participant recruitment will follow a consecutive sampling method, enrolling individuals in the order they present until the target sample size is reached.
Participants and recruitment
Sixty participants will be recruited from the inpatient and outpatient psychiatry departments of The Second Xiangya Hospital of Central South University and The Second People’s Hospital of Dali Bai Autonomous Prefecture, China, between 1 August 2023 and 1 June 2026, according to the anticipated schedule. Eligible participants must meet the diagnostic criteria for schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Patients who exhibit weight gain after adding the prescription dosage of antipsychotics or during a stable medication period will be enrolled. The types and dosages of antipsychotics will remain unchanged during the intervention. The principal investigator will provide information about the study, including its purpose, procedures and potential risks, to both the participants and their caregivers. Written informed consent (see online supplemental file) will be obtained voluntarily before enrolment. The detailed inclusion and exclusion criteria are presented in box 1.
Supplemental material
Inclusion and exclusion criteria
Inclusion criteria
Age between 18 and 40 years old.
Meeting the diagnostic criteria for schizophrenia in DSM-5.
BMI ≥25 kg/m2 or over 10% weight gain after taking antipsychotics during the last year.
Not receiving TMS therapy in the past month.
Using no more than two antipsychotic medications (including olanzapine, haloperidol, amisulpride, asenapine, risperidone, paliperidone, clozapine, quetiapine, iloperidone, chlorpromazine, sertindole and zotepine) and not using antidepressants, mood stabilisers and other drugs, but allowing short-term use of benzodiazepines, benzhexol and propranolol.
Signing the written informed consent voluntarily.
Exclusion criteria
Other severe mental illnesses, intellectual disability, dementia and severe cognitive impairment according to the diagnostic criteria of ICD-10 or DSM-5.
Abnormal brain structure or function owing to any major physical disease, neurological disease, traumatic brain injury, etc.
Metallic implants, pacemakers, epilepsy history or other contraindications to TMS.
Suicidal thoughts or behaviours.
Alcohol or substance abuse.
Pregnant or lactating women.
Other contraindications to MRI.
Received regular MECT or weight loss therapy in the latest month.
Other abnormal examination results considered by the researchers to be inappropriate for inclusion.
BMI, body mass index; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; ICD-10, International Classification of Diseases 10th Revision; MECT, modified electric convulsive treatment; TMS, transcranial magnetic stimulation.
Randomisation and blinding
Participants will be equally recruited from the two centres. A study collaborator, independent of both the study teams of the two centres, will generate two randomisation lists with unique codes using SAS OnDemand for Academics (https://welcome.oda.sas.com/home). Participants will be randomised into either the active iTBS group or the sham control group in a 1:1 ratio according to block randomisation. The study collaborator managing the online randomisation database will create and deliver opaque sealed envelopes containing each participant’s specific randomisation identification number and code of assigned treatment group to treatment providers. These providers will not be involved in any other steps of this trial beyond administering the intervention. To ensure blinding, patients, clinical evaluators, data analysts and other study personnel will remain unaware of treatment allocations and will be asked not to discuss group assignments with one another.
Intervention protocol
All participants will undergo structural MRI scans prior to initiation of the stimulation protocol. The iTBS treatments will be delivered with a Mag-TD stimulator equipped with a B65 fluid-cooled coil (Yiruide, Wuhan, China). The left DLPFC will be localised using scalp measurement, with the target positioned 5 cm anterior to the motor cortex, a method widely applied in published research.20 28 Previous research has indicated that multiple daily sessions of rTMS, referred to as accelerated rTMS, may elicit a more rapid therapeutic response compared with a single daily session of rTMS when identical numbers of sessions or stimulation pulses are applied.29 Moreover, an RCT has shown that accelerated cTBS targeting M1, administered for five sessions daily for 5 days, could effectively facilitate weight management in patients with schizophrenia.26 An animal study has also found that the effects of accelerated TBS on LTP depend on treatment intervals, with a 60 min intersession interval showing a cumulative effect on neuroplasticity through synaptic potentiation.30 Thus, we will develop an accelerated iTBS protocol to attain therapeutic efficacy in a short timeframe while simultaneously minimising treatment burden on patients. Five daily iTBS sessions, with each session consisting of 600 pulses patterned with 5 Hz bursts and with each burst containing three pulses at 50 Hz over 3 min, will be delivered at fixed time points with 1-hour intervals over 5 consecutive days. The stimulation intensity will be set at 90% of the resting motor threshold (RMT), identified as the minimum stimulus required to elicit at least 5 out of 10 contractions of the right abductor pollicis brevis muscles when stimulating the left motor cortex at baseline.
Sham stimulation will be delivered to the control group using a sham coil with identical parameters, which can produce noise and vibratory sensation similar to real stimuli while lacking neurophysiological effects, thereby ensuring effective blinding.
Routine therapy
This trial will be conducted as a supplement to routine medication. Comprehensive information on concurrent therapy, including medication type, dosage, duration and side effects, will be recorded on case report forms (CRFs). Patients will be withdrawn from the study once they adjust or switch antipsychotic regimens before finishing all follow-up visits. Additionally, administration of mood stabilisers, antidepressants, antiepileptic drugs and high-dose benzodiazepines (a dose of more than 1 mg of lorazepam daily) will not be permitted during the study.
Assessments
All participants will undergo primary and secondary outcome assessments at four time points, including baseline (V0), after 5 days of treatment (V5), and at 2-week (V6) and 4-week (V7) follow-up following the completion of the intervention. Outpatients will be required to visit the hospital for treatments during the 5-day intervention period from V1 to V5. A detailed summary of the evaluation measures is provided in online supplemental table S1.
Supplemental material
Primary outcome
The primary outcome measure is the change in weight gain, as assessed by BMI from V0 to V5, V6 and V7. Additionally, waist circumference and abdominal circumference will be recorded at the same time.
Secondary outcomes
Appetite assessment
The key secondary outcome is the change in appetite after iTBS treatments compared with the control group from baseline to V5, V6 and V7. Appetite will be assessed 2 hours after dinner using the revised 21-Item Three-Factor Eating Questionnaire (TFEQ-R21), the Food Cravings Questionnaire-Trait (FCQ-T), the Food Cravings Questionnaire-State (FCQ-S) and the Visual Analogue Scale (VAS).
The TFEQ-R21 is a self-report, 4-point Likert scale designed to measure three dimensions of eating behaviour covering cognitive restraint, uncontrolled eating and emotional eating.31 This questionnaire has been used in obese and non-obese populations across different languages, demonstrating sufficient validity.31–34 The FCQ-T is a 6-point Likert scale that measures individuals’ stable food craving traits across nine factors, including intentions, anticipation of positive reinforcement, anticipation of negative reinforcement, lack of control, preoccupation with food, hunger, cues and emotional experiences of eating, with 39 items over time and in different contexts.35 The internal consistency coefficient of the full scale was 0.97, and the alpha coefficients of the subscales ranged from 0.81 to 0.94.36 The FCQ-S is a 5-point Likert scale that measures the intensity of momentary food craving, with high sensitivity to treatment effects and exposure to food cues.35 The VAS will be used to assess subjective appetite sensations, covering hunger, satiety, desire to eat and overeating. VAS scores range from 0=‘not at all’ to 10=‘extremely’, consistent with our previous study.8
Clinical symptom assessment
Changes in clinical symptoms will be assessed by the Positive and Negative Syndrome Scale (PANSS), evaluating the severity of schizophrenia symptoms37; the Calgary Depression Scale for Schizophrenia (CDSS-C), rating depression severity in patients with schizophrenia38; and the Clinical Global Impressions (CGI) scale, quantifying the overall psychopathology severity and improvements from baseline to V7.39
Neurocognitive assessments
Behavioural tasks will be used to measure changes in cognitive control, reward evaluation and decision-making with regard to food cravings from V0 to V5, V6 and V7.
The cognitive control referring to response inhibition will be evaluated by the SST based on current research exploring the correlation between food intake and inhibitory control.40 41 In the go task, the participants will be required to press a left-hand button when a left-pointing arrow appears on the screen and press a right-hand button when a right-pointing arrow is displayed. In the SST, the patients will be asked to withhold their responses when the arrow’s colour changes after a variable delay.42 Key parameters will be reported as outcomes of this task, including the mean go reaction time, stop signal mean reaction time, stop signal delay, target accuracy, go trial accuracy and no go accuracy.
The DDT will be applied to assess impulsiveness in decision-making. Participants will be presented with hypothetical choices between smaller immediate rewards and larger delayed rewards across different lengths of time intervals and amounts of rewards. The time points when delayed rewards are regarded as equalling the immediate rewards can be identified to determine the discounting rate, which can be calculated using the area under the curve.43
The Chinese version of the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery will be used to assess cognition, including speed of processing, attention-vigilance, working memory, verbal learning, visual learning, reasoning, problem-solving and social cognition from baseline to V7.44
Laboratory examination
Fasting blood tests will be conducted to assess glycolipid metabolic indicators, appetite-regulating factors and neurotransmitters at V0, V5 and V7. Glycolipid metabolic indicators to be measured include fasting blood glucose, insulin, glucagon, glycosylated haemoglobin, triglycerides, total cholesterol, total bile acids, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. The appetite-promoting factors to be analysed include adiponectin, ghrelin, cannabinoid and agouti-related peptide, while the appetite-suppressing factors include leptin, glucagon-like peptide-1, prolactin, cholecystokinin and pro-opiomelanocortin. Additionally, several neurotransmitters will be assessed, including serotonin, dopamine, gamma-aminobutyric acid, glutamate, β-endorphins, norepinephrine and epinephrine.
Bacterial DNA extracted from faecal samples at V0 and V7 will be analysed to measure changes in gut microbiota diversity, abundance and function in modulating the appetite.
MRI scans
The MRI will be performed using a GE Signa Excite 3.0T MRI scanner at the radiology department of The Second Xiangya Hospital, Central South University and The Second People’s Hospital of Dali Bai Autonomous Prefecture. Participants will undergo MRI scans 3 hours after dinner at baseline (V0), the immediate end of all treatments (V5) and 4 weeks after the intervention completion (V7). Participants will be asked to remain relaxed with their eyes closed and wear a sponge headset to reduce head movement during the scan. The whole MRI scan will take approximately 30 min, comprising the following sequences:
High-resolution T1-weighted imaging using the magnetisation prepared rapid gradient echo sequence to detect grey matter volume, white matter volume and cortical thickness.
Arterial spin labelling pulse sequences to quantify the cerebral blood flow (CBF).
Functional MRI (fMRI) based on the blood oxygen level-dependent contrast to detect brain function, covering neuronal activity regional homogeneity, fractional amplitude of low-frequency fluctuations, network homogeneity and functional connectivity.
Safety assessments
Current guidelines have proposed safety concerns regarding TBS, which have been approved by the US Food and Drug Administration in 2018, suggesting that TBS administered at 80%–100% of the RMT is safe, given the low risk of epileptic seizure.45 Recent studies investigating accelerated iTBS for treatment-resistant major depression further ascertain that administering 20 iTBS sessions targeting the DLPFC over 4 successive days is safe and well tolerated.46 47 Although some studies have reported transient adverse effects, such as headaches, scalp pain, dizziness and other discomforts, these symptoms typically disappear within 3 days during the intervention.48 Thus, the stimulation intensity will start at a tolerable level and gradually increase to the target intensity within the first five sessions. Moreover, an adverse event record forum will be used to detect any side effects following each stimulation session. Any severe adverse events will be reported to the ethics committee to assess the necessity for withdrawal.
Sample size calculation
Based on a published study assessing the effectiveness of rTMS in people with obesity, a total of 60 participants is required to detect a between-group mean difference of 1.17 in BMI, assuming an SD of 1.4 at the 4-week follow-up after completing the intervention at 80% power with a two-sided p value of 0.05 and 20% dropout rate.21 The correlation coefficient between preintervention and postintervention measurements is estimated to be 0.5. The sample size calculation is conducted using Power Analysis and Sample Size (PASS V.15.0.5) statistical software.
Data management and statistical analysis
Data on basic information, general examination, clinical symptoms, appetite assessment and side effects will be recorded using paper-based CRFs. Cognitive, MRI and laboratory data will be stored in electronic files using software with built-in data protection measures to prevent unauthorised access or disclosure. All data will be collected accurately under the supervision of a data manager authorised by the principal investigator.
Statistical analysis will be performed using SPSS V.26. Between-group demographic data will be compared using t-tests and χ2 tests. Descriptive statistics of clinical measures will be expressed as mean±SD for continuous variables, while categorical variables will be presented as ratios. Efficacy analysis will be conducted on a modified intention-to-treat population who receive over 80% of treatment sessions, have baseline data and complete at least one postintervention primary outcome assessment. Mixed models for repeated measures will be used to assess changes in scores on TFEQ-R21, FCQ-T, FCQ-S and VAS from baseline to 4 weeks after the end of all treatments. Models include group, time, weight, duration of medication, group-by-time interactions and baseline score-by-time interactions as fixed effects, with baseline appetite scores as a covariate. Changes in weight, BMI, PANSS, CDSS-C, CGI, DDT, SST and neuroendocrine regulators will be assessed with generalised linear mixed models. T1-weighted MRI data will be analysed using the Computational Anatomy Toolbox (CAT12) for Statistical Parametric Mapping (SPM12) in MATLAB, following normalisation to the Montreal Neurological Institute standard space. CBF data will be analysed using Arterial Spin Labelling Perfusion MRI Signal Processing Toolkit (ASLtbx) and SPM12 for image processing. fMRI data will be processed using SPM12, DPABI V.2.3, GRETNA V.2.0.0 and other toolkits. A two-sample t-test based on a general linear model will be used to compare structural and functional MRI differences between groups. Analysis of covariance, followed by post-hoc paired samples t-tests, will be used to compare within-group differences in MRI data. The results will be corrected for multiple comparisons using the false discovery rate method, and brain regions with significant differences (number of voxels >100 and p<0.05) will be defined as regions of interest (ROIs). MRI indices of ROIs will be extracted for correlation analysis with appetite, BMI, clinical symptoms, CBF, cognitive control, metabolic indices and gut microbiota using logistic regression. Spearman correlation analysis will be used to identify the relation between appetite, BMI, cognitive indices, metabolic factors and flora.
Quality control and study progress
All the staff involved in this study will receive standardised training in the technical skills required for various trial procedures, including clinical assessment, stimulation administration, data recording and storage, statistical analysis, etc. To guarantee data integrity, validity and reliability, all observed outcomes will be systematically reviewed, and quality control will be implemented at each stage of data processing. Participant recruitment will start in August 2023, and the results are anticipated by August 2026.
Patient and public involvement
The initial framework of this trial is designed by the authors, and the principal investigator subsequently refined specific details after collecting feedback from patients with schizophrenia and their caregivers. To enhance the feasibility and clarity of the study procedures, five patients with schizophrenia with increased appetite were invited to complete the entire protocol before formal recruitment. Their suggestions regarding improving the clarity of interview questions and optimising the testing procedures, including making the instructions for questionnaires and behavioural tasks more concise and understandable, were carefully considered and incorporated as necessary. The findings of this trial will be released to all the participants.
Ethics and dissemination
The protocol has been approved by the National Clinical Medical Research Center Ethnic Committee of The Second People’s Hospital of Dali Bai Autonomous Prefecture (no: 2023YN3) and The Second Xiangya Hospital (no: 2024K008). All research processes adhere to the principles of Good Clinical Practice/International Council for Harmonisation guidelines. The trial has been registered at ClinicalTrial.gov (NCT05783063). Before screening for eligibility, the researchers will explain the research background, purpose, group assignment, treatment parameters, follow-up schedule, measurements, potential risks and benefits, and information confidentiality to the participants and their legal representatives. Written informed consent will be obtained prior to enrolment. Patients who decline enrolment or choose to withdraw from the study will receive clinical care as usual. The findings of this study will be disseminated through peer-reviewed international publications, as well as posters or presentations presented at national and international conferences.
Discussion
Weight gain and increased appetite induced by antipsychotics remain a significant challenge in managing patients with schizophrenia. Recently, rTMS targeting DLPFC has been suggested as a promising, well-tolerated therapeutic option. Several studies have found that 10 Hz rTMS over the DLPFC for 4–20 sessions exhibits a certain impact on obesity in the general population19 20 and patients with schizophrenia.23 Furthermore, accelerated cTBS, delivered over 25 sessions within 5 days, has been shown to prevent weight gain in schizophrenia, revealing its efficiency advantage over conventional HF-rTMS.23 However, further optimisation in both treatment efficiency and effect size is still desirable.
This is the first randomised, sham-controlled trial designed to determine the effectiveness of iTBS in reducing the excessive appetite and weight gain induced by antipsychotics. In addition, the study will confirm the correlation between appetite alterations and changes in brain structure, cerebral perfusion, brain function, cognitive control, flora and neuroendocrine regulators. These findings will provide insight into the cognitive control mechanisms underlying appetite regulation. Moreover, we will establish a clinical model to predict the efficacy of iTBS in appetite regulation, providing valuable guidance for optimising treatment strategies in patients with schizophrenia who experience significant weight gain after taking antipsychotics. However, the small sample size limits the power to fully assess efficacy.
Crucially, positive outcomes will support the development of a comprehensive, multimodal treatment framework that integrates pharmacological and non-pharmacological interventions. This strategy might address unmet clinical needs, including metabolic disturbances, cognitive impairments and negative symptoms, in patients with schizophrenia. Current studies have shown that combining non-invasive brain stimulation with evidence-based psychosocial interventions such as aerobic exercise, cognitive remediation and family intervention might effectively address the negative and cognitive symptoms in schizophrenia.49 This study contributes to the formulation of innovative, combined treatment models in the future, thereby providing a more comprehensive approach to schizophrenia management.
Ethics statements
Patient consent for publication
Acknowledgments
We thank all the staff, experts and participants engaging in this study.
References
Footnotes
YQ and JY are joint first authors.
YQ and JY contributed equally.
Contributors JH, RW, ZD and CY designed the study. JinY, JunY and TZo will lead the recruitment and the data collection process. YQ and ZT will conduct the statistical analysis. YQ, BX, HC, JL, TZh and YS will contribute to the interpretation of the results. YQ wrote the first draft of the manuscript. JH edited and revised the manuscript. JH is the guarantor of the paper. All authors have approved the submission of the final manuscript.
Funding This work was supported by the Science and Technology Innovation Program of Hunan Province (no: 2024RC3055), the National Natural Science Foundation of China (grant no: 82271545) and the National Key Clinical Specialty Scientific Research Project of The Second Xiangya Hospital, Central South University (no: 19).
Competing interests JH received a research grant from the National Key Clinical Specialty Scientific Research Project of The Second Xiangya Hospital, Central South University.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.