Article Text
Abstract
Introduction New-onset supraventricular arrhythmia (NOSVA) is the most common arrhythmia in patients with septic shock and is associated with haemodynamic alterations and increased mortality rates. With no data available from randomised trials, clinical practice for patient management varies widely. In this setting, rate control or rhythm control could be beneficial in limiting the duration of shock and preventing evolution to multiorgan dysfunction.
Methods and analysis The Control Atrial Fibrillation in Septic shock (CAFS) study is a binational (French and Belgium), multicentre, parallel-group, open-label, randomised controlled superiority trial to compare the efficacy and safety of three management strategies in patients with NOSVA during septic shock. The expected duration of patient enrolment is 42 months, starting from November 2021. Patients will be randomised to receive either risk control (magnesium and control of risk factors for NOSVA), rate control (risk control and low dose of amiodarone) or rhythm control (risk control and cardioversion using high dose of amiodarone with external electrical shock if NOSVA persists) for 7 days. Patients with a history of SVA, NOSVA lasting more than 48 hours, recent cardiac surgery or a contraindication to amiodarone will not be included. We plan to recruit 240 patients. Patients will be randomised on a 1:1:1 basis and stratified by centre. The primary endpoint is a hierarchical criterion at day 28 including all-cause mortality and the duration of septic shock defined as time from randomisation to successful weaning of vasopressors. Secondary outcomes include: individual components of the primary endpoint; arterial lactate clearance at day 3; efficacy at controlling cardiac rhythm at day 7; proportion of patients free from organ dysfunction at day 7; ventricular arrhythmia, conduction disorders, thrombotic events, major bleeding events and acute hepatitis related to amiodarone at day 28; intensive care unit and hospital lengths of stay at day 28.
Ethics and dissemination The study has been approved by the French (Comité Sud-Ouest et Outre-Mer II, France, registration number 2019-A02624-53) and Belgian (Comité éthique de l’hôpital Erasme, Belgium, registration number CCB B4062023000179) ethics committees. Patients will be included after obtaining signed informed consent. The results will be submitted for publication in peer-reviewed journals.
Trial registration number NCT04844801.
- Adult intensive & critical care
- Cardiovascular Disease
- INFECTIOUS DISEASES
- Clinical Protocols
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study is a binational, multicentre, parallel-group, open-label, randomised controlled superiority trial comparing head-to-head risk control, rate control and rhythm control in patients with new-onset supraventricular arrhythmia during septic shock.
Pragmatic design comparing three common strategies in an intention-to-treat approach.
Limitation: not blinded.
Introduction
Background and rationale
New-onset supraventricular arrhythmia (NOSVA) (including atrial fibrillation, atrial flutter and atrial tachycardia) is reported in 40% of patients with septic shock and is associated with haemodynamic alterations and increased mortality.1 2 Efforts to determine the most effective haemodynamic management strategy in this setting are therefore important. In a recent preliminary study from our research team, successful cardioversion of NOSVA in patients with sepsis seemed to be associated with a better prognosis.3 However, because no randomised clinical trial data are available, there is no consensus regarding the best management strategy for NOSVA during septic shock, which has led to major variations in practice.1 2 4–7 Three treatment strategies are commonly used: (1) control of modifiable risk factors for NOSVA without using antiarrhythmic drugs (risk control);8 (2) control of heart rate with the use of antiarrhythmic drugs, often with low dose amiodarone (rate control);9–11 (3) cardioversion using antiarrhythmic drugs (often with high doses of amiodarone) and/or electrical cardioversion (rhythm control).10 11
Rhythm control may improve haemodynamics (by restoring diastolic function and decreasing cardiac metabolic demand), reduce thromboembolic risk and minimise exposure to anticoagulants. Rate control limits the potential adverse effects of high-dose amiodarone and/or electrical cardioversion, while still improving haemodynamics. The risk control strategy minimises the adverse effects of amiodarone while still resulting in conversion of NOSVA in some patients.
Determining the efficacy and safety of these three strategies may provide valuable information to improve clinical decision-making and resource utilisation for this highly prevalent condition. Therefore, we will conduct a multicentre, parallel group, open-label, randomised controlled superiority trial to compare head-to-head risk control, rate control and rhythm control in this setting.
Hypotheses
Our hypotheses are as follows: (1) compared with risk control, rate control and rhythm control each improve haemodynamics, thus decreasing shock duration and mortality; (2) rhythm control outperforms rate control in this setting.
Objectives
Primary objective
The main objective is to compare the efficacy of the three strategies (risk control, rate control and rhythm control) in reducing mortality and duration of shock in septic patients with NOSVA.
Secondary objectives
Secondary objectives are to compare the benefit and risks of the three strategies in terms of haemodynamics, organ dysfunction, morbidity, mortality, safety (including thrombotic events, bleeding events and serious adverse events related to amiodarone and electrical cardioversion) and net clinical benefit.
Methods and analysis
Trial design
This is a binational, multicentre, parallel-group, open-label, randomised controlled superiority trial in patients with NOSVA during septic shock. The trial protocol follows the Standard Protocol Items: Recommendations for Interventional Trial (SPIRIT) reporting guidelines.
Study setting
The study will be conducted in 28 intensive care units (ICUs) in 23 hospitals in France and five hospitals in Belgium (list of study sites in online supplemental file appendix A).
Supplemental material
Eligibility criteria
Inclusion criteria
Adult patients (age ≥18 years) admitted to the ICU will be eligible as soon as they meet all of the following criteria:
Septic shock, defined by the association of the following criteria:
Documented or suspected infection, with initiation of antibiotic therapy.
Initiation of vasopressors (norepinephrine or epinephrine) for at least 1 hour to maintain the mean arterial pressure >65 mm Hg.
NOSVA (including atrial fibrillation, atrial flutter and atrial tachycardia) with heart rate ≥110 beats per minute lasting at least 5 min.
Member of a social security system.
Written informed consent (patient, next of kin or emergency situation).
Exclusion criteria
Patients presenting any of the following criteria will not be included:
Refractory shock, defined as a dose of norepinephrine base or epinephrine base >1.2 µg/kg/min.
Heart surgery or heart transplant in the previous month.
Aortic or mitral valve mechanical prosthesis, significant mitral stenosis (mitral surface <1.5 cm2).
Congenital heart disease other than bicuspid aortic valve, atrial defect or patent foramen ovale.
History of SVA before septic shock, defined as paroxysmal SVA with long-term antiarrhythmic and/or therapeutic anticoagulation or permanent SVA.
NOSVA lasting more than 48 hours (or more than 24 hours under vasopressor therapy); the patient can still be included if transoesophageal echocardiography (under mechanical ventilation) excludes intracardiac thrombus and the patient can receive therapeutic anticoagulation.
Electrical cardioversion or use of amiodarone or another bradycardic drug (beta-blocker, bradycardic calcium channel blocker, digitalis or flecainide) within the 6 hours preceding inclusion.
Contraindication to amiodarone: history of serious adverse event, lung disease or hyperthyroidism related to amiodarone, PR interval >240 ms, severe sinus node dysfunction with no pacemaker, second-degree or third-degree atrioventricular block with no pacemaker, corrected QT interval (QTc) >480 ms, known or treated hyperthyroidism, hypersensitivity to iodine, amiodarone or to any of the excipients, severe hepatocellular insufficiency (prothrombin rate <20%) or diffuse interstitial lung disease.
Serum potassium <3 mmol/L.
Pregnancy or breast-feeding.
Moribund or death expected from underlying disease during the current admission.
Patients deprived of liberty and persons receiving institutional psychiatric care.
Participation in another interventional trial on septic shock and/or rhythm disorder.
Intervention
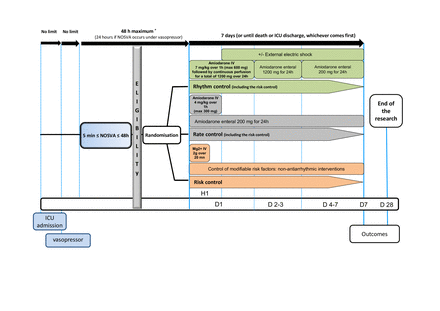
The experimental plan is shown in figure 1. After verification of the eligibility criteria, the patient will be enrolled and randomised (day 1) as soon as possible and within 48 hours from the onset of NOSVA (or 24 hours, if NOSVA occurs when receiving vasopressor treatment). Patients should then immediately receive the allocated strategy for 7 days (or until death or ICU discharge, whichever comes first). The risk control strategy will consist of (1) intravenous bolus of 2 g magnesium sulfate over 20 min (if creatinine clearance >30 mL/min) and (2) control of risk factors for NOSVA, such as hypovolaemia and metabolic disorders (details in table 1). The rate control strategy will consist of (1) control of NOSVA risk factors as described above and (2) ‘low dose’ amiodarone (details in figure 1). The rhythm control strategy will consist of (1) control of NOSVA risk factors as described above, (2) ‘high dose’ amiodarone (details in figure 1) and (3) if NOSVA persists, electrical cardioversion in sedated patients receiving invasive mechanical ventilation (modalities according to the European Society of Cardiology (ESC) guidelines;12details in figure 1). Details of the three strategies according to rhythm and haemodynamic evolution are given in figure 2. In all groups, therapeutic anticoagulation is recommended in the absence of contraindications if NOSVA persists more than 48 hours; modalities of therapeutic anticoagulation will be left to the discretion of the attending physicians. After day 7 (or discharge from the ICU, whichever comes first), NOSVA management will be left to the discretion of the attending physician. All patients will be observed until day 28.
Study schema. D, day; ICU, intensive care unit; IV, intravenous; Mg2+, magnesium; NOSVA, new-onset supraventricular arrhythmia. * If NOSVA > 48h, the patient can still be included if transoesophageal echocardiography (under mechanical ventilation) excludes intracardiac thrombus and the patient can receive therapeutic anticoagulation.
Non-antiarrhythmic interventions for the risk control strategy8
Schema of strategies for each group according to the evolution of the randomised patients. EES, external electrical shock; NOSVA, new-onset supraventricular arrhythmia.
In all groups, current recommendations for the management of septic shock will be followed.13
Criteria and procedures for premature withdrawal of a participant from the study
In compliance with the conventional management of patients with NOSVA during septic shock, the rate control and rhythm control strategies will be discontinued if one of the following occurs:
Ventricular arrhythmia: torsade de pointe, sustained ventricular tachycardia, ventricular fibrillation.
Conduction disorders: severe bradycardia (<50 beats per minute), second-degree or third-degree atrioventricular block, sinus dysfunction (significant sinus pause of at least 3 s), need for a pacemaker, QTc prolongation >480 ms.
Acute hepatitis related to amiodarone, defined by a significant (10-fold) increase in transaminases (hepatic cytolysis), as compared with values before the first dose and with no other identified cause for hepatitis.
Hyperthyroidism, as defined by a thyroid-stimulating hormone concentration <0.1 mIU/L in the blood sampled before amiodarone initiation.14
Follow-up visits
The trial follow-up visits will be on days 2–7 and day 28.
Endpoints
Primary endpoint
The primary endpoint is a hierarchical endpoint assessed at day 28 and includes all-cause mortality and the duration of shock. The Finkelstein-Schoenfeld method is based on the principle that each patient in the clinical trial is compared with every other patient within each stratum in a pairwise manner. The pairwise comparison proceeds in hierarchical fashion, using all-cause mortality, followed by the duration of septic shock when patients cannot be differentiated on the basis of mortality. This method gives a higher importance to all-cause mortality.15 16
The duration (days) of septic shock is defined as the period from randomisation to successful weaning of vasopressors (patient alive with no reintroduction during the first 48 hours after discontinuation).
Secondary endpoints
Secondary endpoints will include the following:
(1) Rhythm control at day 7
Number of patients with sinus rhythm.
Number of days alive with sinus rhythm.
Number of days alive with NOSVA and heart rate <110 beats per minute.
Proportion of patients receiving therapeutic anticoagulation after randomisation.
(2) Morbidity, mortality and organ function
Duration of septic shock at day 28.
Proportion of patients alive and free from vasopressors at day 7 (or discharge or death, whichever comes first).
Arterial lactate clearance at day 3.17
Proportion of patients alive and free from organ dysfunction at day 7 (or discharge or death, whichever comes first). Organ dysfunction is defined as a sequential organ failure assessment score ≥3 for the following organs: cardiovascular, renal, neurological, hepatic, respiratory or coagulation.18
Length of ICU stay at day 28.
Length of hospital stay at day 28.
All-cause deaths at day 28.
(3) Safety
Arterial thrombotic events, including ischaemic stroke and non-cerebrovascular arterial thrombotic event.19
Major bleeding events are defined as bleeds that meet at least one of the following criteria: bleeding in a critical area or organ (eg, intracranial, intraspinal, intraocular and retroperitoneal); bleeding requiring surgical, endoscopic or endovascular haemostasis action; a life-threatening bleed; a fatal bleed.20
Serious adverse events related to amiodarone or to magnesium, including ventricular arrhythmia, conduction disorders and acute hepatitis related to amiodarone as described above.
Serious adverse events associated with electrical cardioversion (for patients receiving electrical cardioversion) including ventricular arrhythmia, conduction disorders and arterial thrombotic events.
(4) Combined efficacy and safety
Net clinical outcome as assessed by the presence of all-cause death, arterial thrombotic event, major bleeding event or at least one serious adverse event related to amiodarone or to magnesium or to electrical cardioversion at day 28.
Sample size and its statistical justification
The sample size was calculated by considering pairwise comparisons between the groups. For each comparison, 1000 samples were simulated using SAS software. Based on data from our two previous studies in patients with NOSVA during sepsis,3 6 the distribution characteristics of the samples were defined according to the following assumptions: (1) 28-day mortality rates of 35%, 30% and 25% for the risk control, rate control and rhythm control groups, respectively; (2) durations of septic shock of 4.9±2.4 days (SD), 3.9±2.4 days and 2.9±2.4 days for the risk control, rate control and rhythm control groups, respectively. Within each sample/pairwise comparison, an individual score was calculated by comparing each patient in one group with all patients in the other groups.15 16 These scores were then compared between groups using a Mann-Whitney/Wilcoxon test in each of the 1000 samples and the p value of each test was recorded. For each pairwise group comparison, the proportion of tests with a p value <0.05 was at least 81% with 80 subjects in each group. Therefore, we expect that having 80 subjects per group will provide a minimum power of 81% to detect a difference in the primary outcome with alpha =5%.
Recruitment
The expected duration of patient enrolment is 42 months starting from November 2021. The study timeline is as follows: (1) 2018: grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique) for academic sponsor (Assistance Publique-Hôpitaux de Paris (AP-HP)); (2) July 2020: approval by independent ethics committees and competent authority; (3) November 2021: start of patient enrolment; (4) 2025: end of patient enrolment, monitoring, cleaning and database lock, blind review to screen for protocol violation; (5) 2025–2026: data analysis, writing of the manuscript and submission for publication.
Allocation of intervention and data management
Randomisation in a 1:1:1 ratio will be prepared by an independent statistician from the Clinical Research Unit before the start of the trial. Randomisation will be stratified by centre and block balanced. The width of the blocks will not be communicated to the investigators. Patients will be randomised using the electronic case report forms (e-CRFs).
Non-identifying data will be entered into the e-CRFs by a trained investigator or research assistant at each centre. Patient follow-up and task schedules are detailed in the study Gantt chart (table 2). The e-CRF was devised by the principal investigator and the scientific supervisor of the study in collaboration with the data manager of the Clinical Research Unit. e-CRFs and a data dictionary (containing coding variables and definitions) will be saved and archived in the Clinical Research Unit and AP-HP secured servers. The computer files used for this research are implemented in compliance with the French (amended ‘Informatique et Libertés’ law governing data protection) and European (General Data Protection Regulation (GDPR)) regulations. The sponsor has already obtained authorisation from the National Commission on Informatics and Liberty (French Data Protection Agency) to process data from this research (Ref.: MLD/MFI/AR2012389). Database quality control will be undertaken by a data manager from the Clinical Research Unit.
Study Gantt chart (task schedule)
Statistical methods
All analyses will be performed by a statistician from the Clinical Research Unit according to the statistical analysis plan prepared before data base lock, using SAS software V.9.4 (SAS Institute, Cary, NC, USA), R software V.4.2.2 and Stata software (V.17; StataCorp).
In compliance with the SPIRIT statement, a flow diagram will describe the progress of the three groups of patients throughout the different phases of the trial (enrolment, allocation, received interventional agents, follow-up and data analysis). The analysis will be performed on an intention-to-treat (ITT) basis. In case of premature interruption or withdrawal from the study, patients will not be substituted. Single imputation will be made for missing values of the primary endpoint, as a failure (ie, death). Sensitivity analysis will be performed in the per-protocol population set (patients as randomised without major protocol violations).
Descriptive analysis
A flow chart will be provided. Descriptive statistical analyses will be conducted on the ITT population to describe general and baseline characteristics. Quantitative variables will be reported as mean (±SD) or median (25th–75th percentiles) according to the distribution of the variable. Qualitative variables will be reported as numbers (%).
Analysis of the primary endpoint
The prespecified primary endpoint will be a ranked composite score that incorporates death and duration of shock, calculated in such a manner that death constitutes a worse outcome than longer duration of shock. Each patient will be compared with every other patient in the study and assigned a score (equality: 0, win: +1, loss: −1) for each pairwise comparison based on who fared better. For example, if one patient survives and the other does not, the first will be attributed +1 and the latter −1 for that pairwise comparison. If both patients in the pairwise comparison survive, the scoring will depend on the duration (days) of septic shock: fewer days earn a score of +1 and more days earn a score of −1. If both patients survive and had the same duration of septic shock, or if both patients die, both will score 0 for that pairwise comparison. For each patient, scores of all pairwise comparisons will be summed to obtain a cumulative score. These cumulative scores will be ranked and compared across the three groups using a non-parametric Mann-Whitney test.16
Analysis of secondary endpoints
Comparisons between randomised groups at given time points will be conducted using Pearson χ2 or Fisher exact tests for categorical variables and using Analysis of Variance or non-parametric Kruskal-Wallis tests for quantitative variables, as appropriate.
For 28-day all-cause mortality, the number of patients with sinus rhythm at day 7, the number of patients free from vasopressors at day 7 and survival without serious adverse events, the calculation of time-to-event endpoints based on follow-up censored data will be employed, taking into account the competing risks of hospital discharge (for mortality evaluation) and death (for the number of patients with sinus rhythm at day 7 and the number of patients free of vasopressor at day 7). Kaplan-Meier survival curves and cumulative incidence curves will be plotted according to treatment group, and Cox models will be used to calculate hazard ratios along with their 95% CIs.
Data monitoring
The trial steering committee (coordinating investigator, scientific supervisor and methodologist) will supervise the progression and monitoring of the study. Clinical research assistants will regularly perform on-site monitoring at all centres to check protocol adherence and accuracy of the recorded data. An investigator at each centre will be responsible for daily patient screening, patient enrolment, adherence to protocol and completion of the e-CRF. Because the three treatment strategies are currently used in routine practice, no data safety monitoring board was required.
Patient and public involvement
Patients and/or the public were not involved in the development of this study.
Discussion
This pragmatic, multicentre, randomised controlled trial will compare the efficacy and safety of risk control, rate control and rhythm control for NOSVA in patients with septic shock. Despite many observational studies showing that tachycardia and atrial fibrillation are key prognostic factors in septic shock,1 2 21 no randomised controlled trial has been conducted to investigate the best strategy to manage NOSVA in this setting.
Strengths of our trial come from its design comparing three distinct commonly used strategies in this setting in an ITT approach, the generalisability embedded in the multicentre design, in which university and non-university hospitals from two countries (France and Belgium) will recruit patients and the carefully selected population. Indeed, in regard to this last point, we will exclude patients with a significant history of SVA (for whom the cardiovascular risk depends in part on their established previous medication regimen for SVA) and patients with a high thrombotic risk (SVA of more than 48 hours, recent cardiac surgery and valvular heart disease classifying SVA as ‘valvular SVA’).22 Importantly, this is an investigator-initiated trial, funded by a grant from the French Ministry of Health with no competing commercial or financial interests.
Our study has several limitations. Because it is an open-label trial, some bias, such as clinical decision-making preferences, is inevitable. Assessment of the duration of septic shock, the second component of the hierarchical primary endpoint, may be subjective, thus liable to performance bias. Reporting bias is unlikely for the primary outcome given that (1) all-cause death is an objective measure and (2) ICU hospitalisation and haemodynamic support are unambiguously supported by medical records.
In summary, the Control Atrial Fibrillation in Septic shock trial is an open label, randomised controlled trial testing the efficacy of three routinely used strategies (risk control, rate control and rhythm control) to improve survival and reduce duration of shock in patients with NOSVA during septic shock. The trial targets a well-selected population, with an appropriate, patient-relevant primary outcome and experimental design, to provide a robust response (best strategy with respect to adverse events). This trial’s results may therefore provide high-quality evidence to inform international recommendations for the optimal haemodynamic strategy in patients with NOSVA during septic shock.
Ethics and dissemination
Ethical approval
The study has been approved by independent ethics committees (Comité Sud-Ouest and Outre-Mer II, France, registration number 2019-A02624-53 and Comité éthique de l’hôpital Erasme, Belgium, registration number CCB B4062023000179).
Consent to participate
Patients will be included after signing written informed consent (online supplemental file appendix B). If a patient is not able to understand the information given in the consent form, they can be included if a next of kin consents. Eligible patients unable to receive information and for whom a substitute decision maker is not present can still be included through a process of deferred consent; after recovery, the patient’s agreement to stay in the trial will be sought.
Supplemental material
Confidentiality
Data will be handled according to the French law on data protection and the European General Data protection regulation. All original records will be archived at the trial sites for 15 years.
Declaration of interest
This study was funded by a grant from the French Ministry of Health obtained in 2018 (Programme Hospitalier de Recherche Clinique). The sponsor is AP-HP (Délégation à la Recherche Clinique et à l’Innovation). Delegation (for Belgian centres) for ethics regulation and monitoring has been agreed by Les Cliniques Universitaires de Bruxelles-Hôpital Erasme.
Access to data
Investigators will make the documents and individual data required for monitoring, quality control and audit of the study available to specified persons in accordance with French law.
Dissemination policy
Findings will be published in peer-reviewed journals and presented at national and international meetings. Communications, reports and publication of the results of the study will be placed under the responsibility of the principal investigator-coordinator of the study and the steering committee. Reporting will adhere to the SPIRIT statement, and rules of publication will follow international recommendations, for example The Uniform Requirements for Manuscripts (International Committee of Medical Journal Editors, April 2010) (SPIRIT checklist, online supplemental file appendix C).
Supplemental material
Ethics statements
Patient consent for publication
References
Footnotes
X @Denis_Doyen
Contributors VL is responsible for the overall content as the guarantor. VL and AMD in collaboration with the other authors designed the study and wrote the manuscript together. AR provided substantial contributions to the conception and design of the study, wrote the statistical analysis plan, and estimated the sample size. VL, CD, SP, DDo, DC, FBa, BS, VP, P-MB, GMu, FBo, PA, NB, JJ, OS, MD, FA, XM, SC, EV, NS, AW, SV, NH, CLB, GC, FC, MP, LH, MF, FT, DDu, GMo, LB, AR and AMD contributed to drafting the work, revising it critically for important intellectual content and approved the final version of the manuscript. VL, CD, SP, DDo, DC, FBa, BS, VP, P-MB, GMu, FBo, PA, NB, JJ, OS, MD, FA, XM, SC, EV, NS, AW, SV, NH, CLB, GC, FC, MP, LH, MF, FT, DDu, GMo, LB, AR and AMD gave their agreement to be accountable for all aspects of the work and ensure the accuracy and integrity of any part of it.
Competing interests AMD reports lectures for Leo Pharma. VL receives advisory board fees from AOP and grants from Leo Pharma unrelated to the present study. XM received fees for lectures from AOP health, Baxter healthcare and Getinge and fees for consulting activities for Baxter healthcare and Getinge.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.