Article Text
Abstract
Objectives We sought to identify groups of high-need high-cost (HNHC) patients with distinct cost trajectories and describe the sociodemographic and clinical characteristics associated with group membership.
Design A population-based retrospective cohort study, using administrative health data.
Setting British Columbia, Canada.
Participants People who were HNHC in 2017, defined as incurring health system costs in the top 5% of the population, and were continuously registered in the Medical Service Plan from January 2015 to December 2019 and alive at the end of the study period.
Outcome measures The primary objective was to identify longitudinal patterns of healthcare costs using group-based trajectory modelling. Adopting a health sector perspective, we conducted person-level costing for hospital episodes, day surgeries, physician services, prescription medications, and home and community care services. The secondary objective was to explore sociodemographic and clinical characteristics associated with group membership using adjusted ORs and 95% CIs from a multinomial logit model.
Results Our final sample comprised 5.4 million British Columbians. In 2017, 224 285 people met our definition of an HNHC and were included in our analysis (threshold: $C7968). We selected a model with five groups. These groups included those with persistently very high costs (44%, mean 5-year total: $C124 622); persistent high costs (32%, mean 5 year total: $C38 997); rising costs (7%, mean 5-year total: $C43 140); declining costs (10%, mean 5-year total: $C30 545); and those with a cost spike (7%, mean 5-year total: $C19 601). Being older, being in the lowest income quintile and having a greater number of comorbid health conditions were associated with increased odds of being in the persistently very-high-cost trajectory group relative to each other group. There was heterogeneity in the association between individual comorbidities and trajectory group membership. Several comorbidities were associated with a statistically significant increase in the odds of being in the persistently very-high-cost group compared with all other groups (eg, diabetes, renal failure), while others were associated with decreased odds (eg, metastatic cancer, alcohol abuse).
Conclusion This study unveils the complex and diverse cost trajectories of HNHC patients in British Columbia, highlighting the necessity for tailored healthcare strategies that address individual patient needs and circumstances. Notably, a high proportion of HNHC patients exhibit persistently high costs over a 5-year period, and available sociodemographic and clinical data are not predictive of group membership. Future research is needed to develop methods for predicting future HNHC patients and to identify evidence-based interventions that can improve patient outcomes and mitigate unnecessary healthcare utilisation and costs.
- Health policy
- Health economics
- Health Care Costs
Data availability statement
Data may be obtained from a third party and are not publicly available. Access to data provided by the Data Stewards is subject to approval but can be requested for research projects through the Data Stewards or their designated service providers. The following data sets were used in this study: Vital Events Deaths; Consolidation File (MSP Registration & Premium Billing); Patient-Centred Measurement; National Ambulatory Care Reporting System; PharmaNet; Medical Services Plan (MSP) Payment Information File; Discharge Abstract Database (Hospital Separations); Home and Community Care dataset. You can find further information regarding these data sets by visiting the PopData project webpage at: https://my.popdata.bc.ca/project_listings/20-073. All inferences, opinions, and conclusions drawn in this publication are those of the author(s), and do not reflect the opinions or policies of the Data Steward(s).
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study identified and characterised five cost trajectories of high-need high-cost patients, defined as those incurring costs in the top 5%, among 5.4 million people in British Columbia, Canada.
We used five calendar years of individual-level health data from comprehensive, population-based health administrative databases.
We excluded those who were not continuously insured and those who died during the study period, meaning that our results may not be generalisable to these populations.
Our costing approach did not capture some healthcare costs, including prescription drugs obtained during a hospital stay and some specialty drugs related to oncology, transplants and renal care.
We relied on health administrative data and thus could not explore the influence of social or behavioural factors on cost trajectories.
Introduction
Healthcare expenditures are highly concentrated in a relatively small proportion of the population.1–4 A systematic review of 55 studies found that over half of all healthcare costs were incurred by just 5% of the population, while nearly a quarter were incurred by 1% of the population.3 This relatively small segment of the population that incurs a disproportionate amount of healthcare costs is known as high-need high-cost (HNHC) patients. A substantial amount of research has focused on describing the characteristics of HNHC patients and their healthcare use.3 HNHC patients often have multiple chronic conditions and functional limitations, and their risk of being HNHC may be amplified by the presence of other co-occurring health conditions, such as mental illness and substance misuse, and social risk factors, such as homelessness, low socioeconomic status (SES) and food insecurity.5–9 The relationship between demographic characteristics and HNHC status is less clear;5 however, increasing age and being near the end of life are associated with an increased risk of being an HNHC patient.3
While HNHC patients are united in their high healthcare use, they are a heterogeneous population.10 Consequently, research has focused on segmenting the population into groups who have similar demographic and clinical profiles.11–13 For example, Wick et al used latent class analysis to describe groups of persistently high-cost patients, defined as those who were HNHC for two consecutive years. Nine groups were identified, including those characterised by severe mental illness or cardiovascular disease, each of which would need different interventions or supports to improve their care and outcomes.13
Given the clinical heterogeneity in HNHC patients, we might expect this to translate into heterogeneity in their costs over time. Selected studies have described the healthcare use and costs among HNHC patients,10 14 15 though most have focused on trajectories of costs during the respective high-cost year or during specific episodes of high-cost care.16 Consequently, the long-term cost trajectories of HNHC patients have not been well characterised.16–20 Understanding the path to (or from) HNHC status and the factors associated with different trajectories may identify opportunities to intervene to improve patient outcomes and reduce costs.15 In this study, we had two aims: (1) identify groups of HNHC patients with distinct cost trajectories, and (2) describe the sociodemographic and clinical characteristics associated with group membership.
Methods
Reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines and the Guidelines for Reporting on Latent Trajectory Studies checklist.21 22
Study design and setting
Our study used five calendar years of data from Population Data BC.23 The data from Population Data BC included longitudinal, person-specific, de-identified health data on British Columbia’s (BC’s) 5.4 million residents. This included data from the Discharge Abstract Database (hospital separations, including day surgeries), National Ambulatory Care Reporting System (emergency department), Medical Services Plan (MSP: fee-for-service physician services), PharmaNet database (prescription medications and medical supplies) and Home and Community Care database (long-term care, assisted living, adult daycare and home care). Our final sample included all British Columbians who were continuously registered in the single-payer public health insurance plan (MSP) from January 2015 to December 2019 and alive at the end of the study period. Additional information on the datasets used in this analysis is available from Population Data BC at https://www.popdata.bc.ca/data.24
Costing
We conducted person-level costing with a health system perspective, following best practice guidelines. For example, inpatient hospitalisations were costed from the Discharge Abstract Database using case-mix costing which involves multiplying the cost of a standard hospital stay by a resource intensity weight.25 Emergency department care was costed by identifying unique visits using the National Ambulatory Care Reporting System database, Discharge Abstract Database and Medical Service Plan database, and multiplying each by an emergency department facility cost for each encounter.26 Home and community care costs were estimated using the Home and Community Care database, which includes multiplying the number of days in care by an estimated cost per day, which is calculated by dividing residential care expenditures by the total number of residential care days. Lastly, physician and prescription drug costs were obtained directly from the Medical Service Plan and PharmaNet databases, respectively.
We began by aggregating costs at the daily level, with costs spanning multiple days (eg, hospitalisation, 30-day prescription) distributed evenly across the period. Costs were then aggregated at the monthly level. This approach has been used previously and helps to assess trends over time.17 Costs were adjusted to 2019 Canadian dollars using the health and personal care component of the Consumer Price Index. We defined HNHC patients as those incurring annual healthcare costs in the top 5% of the population. In each calendar year of the study period, every individual in our sample was classified as either HNHC or not.
Statistical analysis
We conducted a descriptive analysis of healthcare costs in our sample over the study period. The standard three-step method identified groups and explored the predictors of class membership.22 The first step identified the number of unique cost trajectory groups using group-based trajectory modelling (GBTM). The second step identified the most likely cost trajectory group for everyone, and the third step used a multinomial logit model to investigate covariates associated with membership in each group.
Descriptive analysis
Descriptive analysis focused on describing overall cost characteristics for HNHC patients. We summarised costs (total and by cost category) using descriptive statistics (mean, SD, median, IQR).
Identification of cost trajectory groups
Trajectory modelling is widely used in clinical research to understand the change in an outcome over time.27 Nagin and Odgers identify three different types of trajectory models, including (1) growth curve modelling (GCM), (2) growth mixture modelling (GMM) and (3) GBTM.27 We chose not to use GCM because it requires an assumption that all individuals in the population follow a similar trajectory,27 which prior research demonstrates is not a reasonable assumption in the current context.16 Both GMM and GBTM allow for groups of the population of interest to follow different trajectories,28 however they differ in that GBTM assumes all individuals within a group follow the same trajectory, whereas GMM includes random effects, which is more computationally intensive, but also captures differences in trajectories among individuals in the same trajectory group.28 We chose GBTM over GMM because our focus was on identifying groups of individuals who share similar cost trajectories and could be targeted with tailored policies or interventions. This does not require understanding heterogeneity among the different trajectory groups.
Here, we implemented GBTM to identify groups with distinct trajectories of quarterly log-costs (PROC TRAJ function in SAS). Our analysis of cost trajectories focused on those who were an HNHC patient in 2017, that is, the middle year of the study period. The focus on HNHC patients from a single calendar year simplified the analysis. Including participants designated as an HNHC patient in any calendar year would result in users with similar cost trajectories (eg, a cost spike) that occur in different years being modelled together. This could dilute trends or result in an inflated number of trajectory groups which reflect differential timing rather than unique trajectories. We began by fitting models that included 2–10 trajectory groups with up to fourth-order polynomials. We did not consider covariates in the model.22 We chose our final model and identified the number of trajectory groups using several criteria, including Akaike information criterion (AIC) and Bayesian information criterion (BIC), log-likelihood and entropy, visual assessment of the trajectory groups to ensure that they were distinct from each other, and by evaluating the posterior probability of group membership to ensure it exceeded a minimal threshold of >0.7.27 We conducted sensitivity analyses to explore the impact of modelling decisions on our results. This included exploring the impact of different samples (eg, HNHC in 2016 and in 2018), threshold definitions of HNHC patients (top 1% and top 10%) and ways of modelling cost (monthly costs and rolling costs). We assumed that resource use and cost data were comprehensive; thus, there were no missing data for our GBTM analysis.
Characteristics associated with trajectory group membership
We explored the association between time-stable sociodemographic and clinical covariates and group membership using a multinomial logit model.27 The model included age, sex, urban area (rural vs metropolitan), neighbourhood income quintile and comorbid conditions as measured using the Elixhauser Comorbidity Index.29 Age, sex, urban area and neighbourhood income quintiles were measured using data from the first year of our analysis (2015). All 30 comorbidities in the index were measured across the 5-year study period (2015–2019) and dichotomised as either present or absent. We also included a variable which counted the total number of comorbidities and categorised individuals as having either 0, 1, 2, 3 or 4 or more comorbid conditions. Model selection was conducted using stepwise backward variable selection, and we chose the best model based on the AIC. As a sensitivity analysis, we also conducted variable selection using stepwise forward variable selection. We assumed that our data on comorbidities were comprehensive (ie, no missingness); however, there were missing demographic data. Given the relatively small proportion of missingness, we conducted a complete case analysis to identify the characteristics associated with trajectory group membership.
Patient and public involvement
Two patient partners (MP and KW) were members of the research team. Their contributions included outlining the research questions, participating in all team meetings, providing feedback on the analysis plan and reviewing and providing critical feedback on the manuscript and other dissemination materials including conference abstracts and presentations. Their feedback was critical in the decision to use the GBTM methodology. They felt it was important to explore the longitudinal cost trajectories of people who transition to or from HNHC status, and that the graphical output provided by GBTM was a more intuitive way to engage with the results.
Results
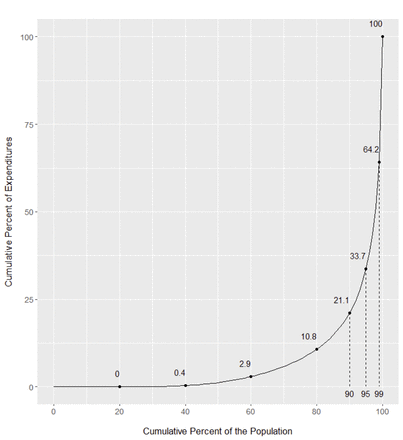
Our final sample comprised 5.4 million persons. The cumulative distribution of healthcare expenditures by the per cent of the population is presented in figure 1. We found that in 2017 approximately 27% of British Columbians incurred no healthcare costs, while the top 10%, 5% and 1% accounted for 79%, 66% and 36% of healthcare expenditures, respectively. Over 224 000 British Columbians met our definition of being an HNHC patient in 2017 (cost threshold to be in the top 5% was $C7968). Of these individuals, 39% were HNHC in 1 year (2017), while 21%, 15%, 10% and 15% were HNHC for 2, 3, 4 and 5 years, respectively. Compared with the BC population, HNHC patients tended to be older, were more likely to be female and to have a higher number of comorbid conditions (see table 1).
Distribution of healthcare expenditures in British Columbia in 2017.
Characteristics of high-need high-cost (HNHC) patients (top 5%) and the British Columbia (BC) general population
Identification of cost trajectory groups
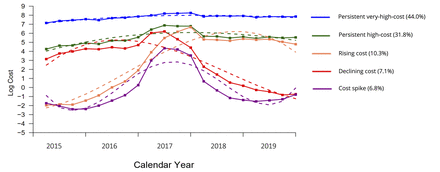
GBTM results for four to seven trajectory groups are presented in the online supplemental figure A1, while model fit statistics are available in online supplemental table A1. We selected a five-group model of cost trajectories. The average and predicted values (including 95% CIs) of the five-group model are available in online supplemental table A2. Figure 2 plots the mean actual (solid line) and predicted trajectories (dashed line) for each group. The groups included (1) persistent very-high-cost (44.0%), (2) persistent high-cost (31.8%), (3) rising cost (10.3%), (4) declining cost (7.1%) and (5) those exhibiting a cost spike (6.8%). All told, over three quarters of HNHC patients were associated with a persistent very-high-cost or high-cost trajectory group. The mean trajectories for each group alongside individual trajectories (1% sample) are presented in online supplemental figure A2 while the model parameters are described in online supplemental table A3. Sensitivity analyses found similar trajectory groups in HNHC patients from the prior or following year (online supplemental figure A3) and for different HNHC thresholds (online supplemental figure A4).
Supplemental material
Actual (solid line) and predicted (dashed line) 5-year cost trajectories from the five-group trajectory model of 2017 high-need high-cost patients (top 5%) in British Columbia, Canada.
Table 2 describes the cumulative cost profile of each of the five trajectory groups over the 5-year study period. On average, those in the persistent very-high-cost trajectory group incurred the highest costs (mean: $C124 622, SD=$C92 700; median: $C85 735, IQR=$C52 305–$C148 838). In contrast, those in the cost-spike trajectory group incurred the lowest costs (mean: $C19 601, SD=$C5181; median: $C13 828, IQR=$C10 976–$C20 112).
5-year costs of high-need high-cost patients (top 5%) by trajectory group
Characteristics associated with trajectory group membership
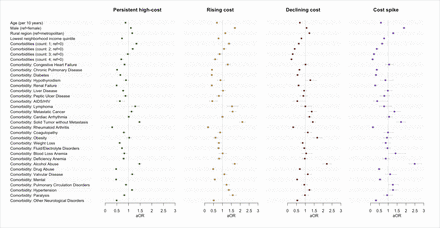
The distribution of observable patient characteristics by trajectory group is available in online supplemental table A4. The results of the multinomial logit model estimated using stepwise backward variable selection (adjusted ORs and 95% CIs) are presented in figure 3 and online supplemental table A5. Of all the variables considered, all but one comorbidity indicator from the Elixhauser index (peripheral vascular disorders) was retained in the final model following stepwise backward variable selection. Membership in the persistently very-high-cost trajectory group was associated with increased odds of being older, living in a metropolitan (rather than non-metropolitan) area, and a neighbourhood with lower average household income. With respect to comorbid conditions, those associated with a persistently very-high-cost trajectory group had increased odds of reporting a greater number of comorbid conditions. There were increased odds of most comorbid conditions in the persistently very-high-cost group relative to the other groups, including the persistent high-cost trajectory group. Some notable results include that the persistently high-cost trajectory group was associated with decreased odds of common ambulatory care sensitive conditions, including congestive heart failure, chronic pulmonary disease and diabetes. In contrast, being in the persistent very-high-cost trajectory group was associated with decreased odds of hypertension, alcohol abuse and metastatic and non-metastatic cancers, relative to the other four trajectories. The overall fit of the model as measured by McFadden’s pseudo-R2, which represents the proportion of variance in the dependent variable that is explained by the independent variables, was 0.1 (Nagelkerke R2=0.16). Our sensitivity analysis using stepwise forward variable selection using AIC resulted in the same model specification as stepwise backward variable selection. Results from the stepwise forward variable selection process are presented as a forest plot in online supplemental figure A5.
Forest plot of factors associated with group membership, relative to the ‘very-high-cost’ trajectory group, from multinomial logit model. aOR, adjusted OR.
Discussion
The study described the 5-year healthcare cost trajectories of HNHC patients in BC, Canada, using comprehensive, population-based administrative databases. We found that HNHC patients exhibit heterogeneous longitudinal cost trajectories; however, three quarters of HNHC patients were associated with either a persistent high-cost or persistent very-high-cost trajectory. This demonstrates that for many HNHC individuals costs remain elevated and stable over time. We found that being in the persistent very-high-cost trajectory was associated with several factors, including older age, lower SES and multimorbidity.
There have been few applications of GBTM to model healthcare costs, often near the end of life and/or in specific clinical populations.17 18 30–34 For example, Teraoka et al used GBTM to explore healthcare costs for older adults in Japan in the final 5 years of life.32 They found that nearly three-quarters of their sample was associated with the two most common trajectories, including persistent-high costs (46%) and persistent medium-to-high costs (26%). We had similar findings despite excluding those who died during the study period. Placona et al explored latent cost trajectories of HNHC Medicare patients.16 Despite being conducted in a different healthcare setting, using a different clustering method (k-means), definition of HNHC (top 10%) and length of analysis (3 years), there were similarities to our findings. For example, Placona et al identified three distinct cost trajectories in four and five group models (persistent high-cost; rising cost; and episodic high-cost) which closely mirror our own findings. They also found a high proportion of patients who were persistently high-cost across the study period (51%). Collectively, these results suggest that persistent high costs are a hallmark of the HNHC population. We found that over 60% of all HNHC patients in BC were under 65 years of age, and 39% had two or more comorbidities, as measured by the Elixhauser comorbidities index. In contrast, Guilcher et al used Ontario administrative data from 2010 to 2011 and found that a smaller proportion of the HNHC population was under 65 (48%) while nearly 59% had eight or more distinct comorbid conditions.35 These discrepancies may reflect differences in the underlying population, time trends or in the case of comorbidities, the specific indices used.
We conducted an analysis to determine which sociodemographic and clinical factors were associated with group membership. We found that being in the persistent very-high-cost group was associated with increased odds of being older and the presence of multiple comorbidities, both factors that have been identified previously.3 5 However, there was heterogeneity in the relationship between specific comorbidities and cost trajectory groups. For example, several comorbidities were associated with a statistically significant increase in the odds of being in the persistently very-high-cost group compared with all other trajectory groups, including chronic obstructive pulmonary disease, diabetes, renal failure, AIDS/HIV and rheumatoid arthritis. Conversely, diagnoses of both metastatic and non-metastatic cancer and alcohol abuse were associated with a statistically significant decrease in odds of being in the persistently very-high-cost group compared with all other trajectory groups.
We found that the overall fit of our multinomial model, as measured by McFadden’s pseudo-R2, was 0.1. Lee et al found similar results when investigating the relationship between demographic and clinical predictors and hospitalisation trajectories among HNHC patients (pseudo-R2 range=0.03–0.07),20 as did Schneider et al, despite focusing on a more clinically homogeneous population (people with advanced breast cancer at the end of life) and having access to patient hospital records which included detailed information on treatment.17 Importantly, McFadden’s pseudo-R2 has been demonstrated to be lower than other commonly used measures of R2, with 0.2–0.4 ‘indicative of extremely good model fits’ and ‘equivalenced to 0.7–0.9 for a linear function’.36 Regardless, the value obtained from our model falls outside this range, suggesting that there are other unknown variables, not included in this analysis, which may predict the cost trajectory groups. Previous research has highlighted the association between social factors and health behaviours and HNHC status.7 8 However, we did not have comprehensive data on these factors. For example, we lacked an indicator for housing insecurity, relied on a proxy for SES (neighbourhood income quintile) and did not have information on self-reported health status, smoking status or level of physical activity.
There are several clinical and policy implications of this work. Our results suggest that a considerable portion of HNHC patients incur persistently high healthcare costs, and these patients have high rates of multimorbidity. For example, nearly a quarter of patients associated with the persistent very-high-cost group had four or more comorbid conditions as measured by the Elixhauser index. There have been relatively few studies evaluating the impact of interventions targeting those with multimorbidity, with those that are available reporting either mixed results.37 That said, we found evidence that several prevalent ambulatory care sensitive conditions (ACSC) were associated with the persistent very-high-cost trajectory group, including chronic heart failure, chronic obstructive pulmonary disease and diabetes. Given that ACSCs are defined as conditions whereby timely and effective primary and outpatient care can reduce the risk of subsequent hospitalisation,38 identifying and targeting individuals who incur persistently high costs for ACSC is one potential area to improve outcomes and reduce costs. Wick et al identified several interventions that could support improving care and outcomes for different high-cost patient populations, including increased access to specialty outpatient clinics for cardiac and pulmonary care, encouraging the use of home haemodialysis for those with advanced kidney disease, and improving community care for those with substance use disorders or severe mental health conditions.13
We also found that lower SES was associated with increased odds of being in the persistent very-high-cost trajectory. This is consistent with previous Canadian research that demonstrated that future high-cost healthcare use is strongly associated with SES, including income, housing, food security and education.7 This emphasises that policies that target the social determinants of health, such as providing income support or access to affordable housing, have the potential to mitigate high healthcare utilisation. Finally, our analysis has highlighted the limitations of administrative health data in capturing information on the social determinants of health, and the importance of improving the collection of these data so we can better understand their influence on health outcomes and healthcare use. This underscores the importance of efforts by groups like the WHO, Healthy People 2030 and Canada Infoway, who are spearheading efforts to improve the routine collection of data on the social determinants of health.39–41
There are several strengths and limitations that warrant consideration. The strength of this study is that it uses health administrative databases, and as such, we have a large, representative sample of the BC population and comprehensive data on resource use and health system costs. Another strength is that we used 5 years of data which provides insight into how people transition to and from HNHC status. Our decision to focus on data from 2015 to 2019 was intentional and based on several key considerations. The COVID-19 pandemic caused unprecedented disruptions in healthcare utilisation and costs, introducing variability that may not reflect stable, long-term cost trajectories. Including pandemic-era data could distort findings by introducing spurious trends that do not accurately represent typical cost patterns among HNHC patients. There are also some limitations. First, we excluded those who were not continuously enrolled in MSP, or those that died during the 5-year study period, meaning that our analysis may not be generalisable to these populations. Second, our costing approach did not capture some costs. For example, the PharmaNet database does not capture prescription drugs obtained during a hospital stay, or those administered through the BC Cancer Agency (ie, oncology medications such as chemotherapy drugs), BC Transplant Society (ie, transplant-related drugs) or BC Renal Agency (ie, drugs for kidney dialysis). Lastly, relying on administrative data alone means we could not explore the relationship between some demographic (eg, race/ethnicity) and social and behavioural factors on cost trajectories. This could be accomplished by using linked survey data; however, this would dramatically reduce the sample size available for analysis.
Conclusions
HNHC patients in BC exhibit heterogeneous longitudinal cost trajectories; however, a high proportion are associated with persistent costs over a 5-year period. Multimorbidity is a hallmark of persistent very-high costs; however, available socio-demographic and clinical data do not predict who is likely to be associated with different cost trajectories. Future work is needed to differentiate between preventable and non-preventable healthcare costs, develop methods for predicting people at risk of becoming HNHC and to identify interventions that can improve outcomes and reduce costs.
Data availability statement
Data may be obtained from a third party and are not publicly available. Access to data provided by the Data Stewards is subject to approval but can be requested for research projects through the Data Stewards or their designated service providers. The following data sets were used in this study: Vital Events Deaths; Consolidation File (MSP Registration & Premium Billing); Patient-Centred Measurement; National Ambulatory Care Reporting System; PharmaNet; Medical Services Plan (MSP) Payment Information File; Discharge Abstract Database (Hospital Separations); Home and Community Care dataset. You can find further information regarding these data sets by visiting the PopData project webpage at: https://my.popdata.bc.ca/project_listings/20-073. All inferences, opinions, and conclusions drawn in this publication are those of the author(s), and do not reflect the opinions or policies of the Data Steward(s).
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the University of British Columbia Research Ethics Board (H20-00029).
References
Footnotes
X @kimchspr, @LLi_1
Contributors LT designed the research and analysis plan in consultation with and using feedback from all the authors. DG and LT conducted the analysis. LT wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version. LT is the guarantor of the work and affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding LT is the Leo Greenawalt endowed professor in health policy and was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship in Patient-Oriented Research. This study was also supported by funding from the EuroQol Research Foundation (164-2020RA) and the BC SUPPORT Unit Health Economcis and Simulation Modelling Methods Cluster.
Disclaimer The funders had no role in the study design; the collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the article for publication
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.