Article Text
Abstract
Objectives Ineffective erythropoiesis, although at a mild degree, could make individuals with beta thalassaemia trait (BTT) vulnerable to folate deficiency. This could be more pronounced in communities where dietary intake of folate is substantially lower. We aimed to assess serum folate levels and dietary folate consumption in individuals with BTT and compare them with healthy matched controls.
Design A case–control study.
Setting This study was conducted between June 2021 and June 2022 at a regional thalassaemia centre in Sri Lanka.
Participants The study includes 100 sets of samples, including a BTT and an age-matched, sex-matched and body mass index-matched healthy individual from the same household in each set, aged between 6 and 25 years.
Primary and secondary outcome measures The primary outcomes were serum folate levels and the daily dietary intake of folate in BTTs and controls. Serum folate levels were determined using a fully automated Cobas immunoassay analyser. The dietary folate intake was determined by recording 24-hour dietary recall recorded over 3 consecutive days, with the average intake calculated.
Results The results suggested that 34% of cases and 24% of controls had serum folate deficiency (<3 ng/mL), while 37% of cases and 49% of controls were at risk (3–5.9 ng/mL) for folate deficiency. Overall, the serum folate level was not significantly different between the cases (mean; 4.88 ng/mL) and the controls (mean; 4.76 ng/mL) (p=0.759). Dietary folate intake was lower than the recommended dietary allowances in both groups, but not significantly different between the groups.
Conclusions There were high levels of folate deficiency in both controls and those with BTT, while those with BTT were no more likely to be folate deficient than the controls. Based on our findings, a policy of indiscriminate folic acid supplementation for all with BTT does not seem rational.
- NUTRITION & DIETETICS
- HAEMATOLOGY
- Case-Control Studies
- Health
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Followed rigorous methodological and reporting guidelines.
The thalassaemia status of all the participants was confirmed prior to the study by high-performance liquid chromatography method.
Both beta thalassaemia traits and healthy controls were assessed for serum folate levels, and the dietary folate intake was estimated using the average of three dietary recall surveys done on consecutive days.
Not measuring red cell folate level and using only serum folate level is a technical limitation of the study.
Even though 3 days of dietary measurements were carried out, the accuracy of the recall is as good as the level of cooperation and interest of the participants.
Introduction
Beta thalassaemia is one of the most common inherited blood disorders in the world, with an estimated 350 million in the world carrying the affected gene. In Sri Lanka, 2.2% of the population are beta thalassaemia traits (BTTs).1 In general, BTTs do not show clinical symptoms, but mild anaemia is usually present. The degree of ineffective erythropoiesis in BTT is clearly much less than that observed in the more severe syndromes, such as non-transfusion-dependent and transfusion-dependent thalassaemia.2
Folate is a naturally found water-soluble vitamin that acts as a coenzyme for many important biochemical reactions, including nucleic acid synthesis, and is required for red blood cell formation.3 Humans and other animals cannot synthesise folate. However, plants and bacteria are capable of synthesising it, which is found in leafy vegetables, egg yolks, legumes, liver and some citric fruits. Dietary folates are derived from the basic structure of folic acid (pteroylglutamic acid).4 The pharmaceutical preparation of folic acid is the synthetic form used in supplements, fortified foods and for treatment. The bioavailability of food folate is around 50% lower than that of folic acid.
The reference ranges of serum folic acid for adults and children are 2–20 ng/mL and 5–21 ng/mL, respectively.5 According to the WHO, a serum folate concentration below 3 ng/mL indicates biochemical deficiency.6 The main cause of folate deficiency is dietary folate deficiency compared with malabsorption and chronic diseases. Although the daily requirement of folic acid is 65–300 µg/day for infants and children and 400 µg/day for adults, multivitamins invariably contain 0.8–1.1 mg of folic acid and this has become the daily supplementation in most instances. However, depending on the disease condition, a dose of 5 mg/day is also recommended.7 8
Folic acid replacement is a standard practice for many diseases, including severe thalassaemia syndromes such as transfusion-dependent and non-transfusion-dependent thalassaemia. Some clinicians recommend lifelong folic acid treatment for BTT, perhaps with the intention to mitigate the effects of mild ineffective erythropoiesis, though the practice is not universal.2 9 Folate deficiency is known to be relatively common in many communities where thalassaemia is also widely prevalent. Studies on folate levels in those with BTT have yielded contradictory results.10 11 Folate deficiency can be high in certain countries, especially in circumstances where the dietary intake too can be compromised.12 Would a practice of universal supplementation of folate for BTTs irrespective of their social circumstances be justifiable, or should such a practice be considered in situations where the community folate deficiency is high?
We designed this study to assess serum folate levels and dietary folate consumption in those with BTT and compared it with healthy matched controls.
Materials and methods
Study design and study population
This study was a case–control study including 100 sets of samples aged between 6 and 25 years. WinPepi software, V.11.65, was used to calculate sample size at the significance level of 5%, power 80%, mean difference of 6 ng/mL and SD of 15 ng/mL for equal number of cases and controls.13 14 The study was conducted between June 2021 and June 2022 at the adult and adolescent thalassaemia centre, Ragama, Sri Lanka. Past records of family screenings conducted at the thalassaemia centre since 2018 were perused to identify families with thalassaemia trait. If there were individuals with thalassaemia trait (case) and a normal healthy sibling (control) in the same household, the individuals or parents/guardians were contacted, and the consent and/or assent was obtained for the study. Only case–control pairs who gave the written consent and satisfied the inclusion and exclusion criteria were recruited for the study. Cases were defined as BTTs without any other acute or chronic disorders. Controls were defined as age-matched, sex-matched and body mass index (BMI)-matched healthy individuals from the same household. The difference in age between the case and the control of each pair was not more than 3 years. None of the subjects were on medications or folic acid supplements. The exclusion criteria include a recent history of folic acid treatment, any other inherited blood disorders and any other chronic diseases.
Data collection and sampling
At the time of recruitment, subjects or parents were interviewed to gather sociodemographic data (age, sex, ethnicity, place of residence and household income) and anthropometric measurements (height and weight) of participants into an interviewer-administered questionnaire. Participants’ medical records were carefully observed for any information on inherited blood disorders or chronic disease conditions. Both cases and controls were analysed for nutrition consumption and serum folate level. The dietary intake of each participant was determined by recording a 24-hour dietary recall of all the foods and beverages they consumed, with portion sizes estimated using household measures on three consecutive days, and the average was calculated. The dietary information of children was obtained by their parents or guardians. Daily dietary intakes of folate and other selected nutrients were determined using a food composition database for Sri Lankan mixed dishes. The blood specimens of the participants were drawn into two tubes; ethylenediaminetetraacetic acid anticoagulated tube and a plain tube, after a 12-hour overnight fast, between 9.00 and 11.00, by venipuncture. Full blood count test of all samples was performed within 4 hours of collection, and the same sample was stored at 4°C and analysed for haemoglobin fractions using the high-performance liquid chromatography method within the same day. Blood samples in the plain tubes were allowed to clot, and serum was separated and stored at 2°C–8°C. The serum folic acid level was determined within 48 hours using a fully automated Cobas immunoassay analyser.
The explanatory variables comprised gender (male and female), ethnicity (Sinhala, Tamil, Moors), place of residence (urban and rural) and family income (<US$82, US$82–US$165, US$165–US$247, US$247–US$330 and >US$330). According to the Sri Lankan BMI classification for adults, its range ≥25 kg/m2 is considered obese, 23–24.9 kg/m2 is considered overweight, 18.5–22.9 kg/m2 is considered normal and <18.5 kg/m2 is considered underweight.15 For the children, sex-specific BMI for age charts available in the Sri Lanka Child Health Development Record were used to determine the obese (BMI more than +2 SD), overweight (BMI between +1 and +2 SD) and wasting (BMI below −2SD).15
Age groups (6–9, 10–11, 12–15, 16–18 and above 18 years) were considered to compare dietary intakes against recommended dietary allowances (RDA) for Sri Lankans.16
The diagnosis of BTT was established on the basis of haemoglobin A2 (HbA2) level>3.5%, and the subjects with HbA2 level below 3.2% were considered normal controls. A serum folate concentration<3.0 ng/mL was defined as folate deficiency, while a serum folate concentration between 3 and 5.9 ng/mL was defined as a possible deficiency.6 According to WHO guidelines, anaemia was defined among different age groups as follows: a haemoglobin concentration (Hb)<115 g/L for children 5–11 years old, an Hb<120 g/L for children 12–14 years old, an Hb<120 g/L for females≥15 years old and an Hb<130 g/L for males≥15 years old.17
However, when determining the full blood count parameters, complete information could be obtained from 94 cases and 91 controls only. Similarly, the prevalence of anaemia and underweight among the control group was determined using data from 98 participants due to missing data.
Statistical analysis
Data were analysed using SPSS V.20.0. Continuous variables were expressed as mean±SD (normal distribution), and categorical variables were expressed in frequency. The student’s t-test and Mann-Whitney U test were used to identify significant differences in anthropometric measurements, full blood count parameters, serum folate levels and dietary intakes between BTT and the control groups. The Pearson correlation coefficient test was used to identify the relationship between five family income levels and certain health/dietary factors of the control group. A p<0.05 involved in this study was considered significant. In the stage of study designing (sampling), demographic/socioeconomic characteristics and anthropometric measurements which would be confounders were controlled by matching cases and controls with nearly similar characteristics. Therefore, the effect of confounders due to the above variables on the final outcomes is likely to be minimum.
Patient and public involvement
Patients and the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Of the 100 pairs of participants in the study, 66 (66%) pairs were females. The current study consisted of case–control pairs from all three main ethnicities living in Sri Lanka, namely Sinhalese (91/100; 91%), Tamil (2/100; 2%) and Muslims (7/100; 7%). Out of 100 pairs, 39 (39%) were from urban areas, while 61 (61%) were dwellers from rural areas. The majority of case–control pairs in the study group (49%; 49/100) had a household income between US$82 and US$165 (table 1), which in comparison with the report of Household Income and Expenditure Survey 2019, Sri Lanka, falls into the poor income (upper range).18 Based on a multivariate analysis of the collected data, none of the variables (demographic/socioeconomic characteristics and anthropometric measurements) were identified as confounders for serum folate level (age, p=0.066; sex, p=0.822; ethnicity, p=0.831; sector distribution, p=0.299; level of income, p=0.412; weight, p=0.060) and dietary folate level (age, p=0.060; sex, p=0.147; ethnicity, p=0.880; sector distribution, p=0.373; level of income, p=0.056; weight, p=0.070).
Demographic characteristics, socioeconomic characteristics and anthropometric measurements of case–control pairs
There were statistically significant differences between BTTs (cases) and controls for all full blood count parameters, including red blood cell count, Hb, mean corpuscular volume, mean corpuscular haemoglobin (MCH), MCH concentration, red cell distribution width and HbA2 (p<0.05) (table 2).
The mean value±SD and differences of full blood count parameters between beta thalassaemia traits and normal controls
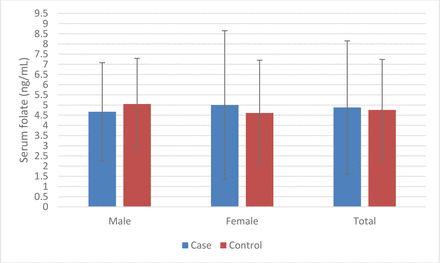
There were no significant differences in mean serum folate levels between BTTs and normal controls (table 3 and figure 1).
Summary of serum folate level in each study group
Mean serum folate levels of cases and controls.
The dietary recall survey did not show significant differences between folate, total energy and protein intake between cases and controls who were from the same household. Dietary folate intakes of all age groups were less than the RDA for Sri Lankans in both cases and controls (table 4).16 The daily average intake of total energy was also less than the RDA in most age groups. Significantly, all the males and females in age groups of 12–15 and 16–18 years showed a daily protein intake less than the RDA except the female cases between 12 and 15 years (online supplemental table).
Supplemental material
The dietary folate intake among cases and controls
Among the non-BTTs (controls), 30/98 (30.6%) were underweight. Of them, 23/30 (76.6%) are females (data are not shown). The prevalence of anaemia among the controls was 17% (n=16). According to the Pearson coefficient correlation analysis, haemoglobin (r(98)=0.36, p=0.004), dietary folate (r(100)=0.60, p=0.000) and protein intake (r(100)=0.71, p=0.000) of the control group show a positive correlation with the family income levels.
Discussion
Our study showed no significant difference in mean serum folate levels in BTTs compared with healthy controls. Notably, quite a high proportion of BTTs as well as the non-trait controls showed serum folate deficiency (34% and 24%), whereas a further 37% of cases and 49% of controls were at risk for folate deficiency. Similar to our data, Gallerani et al did not find significant differences in serum folate levels between BTTs and normal controls in Italy.10 However, they had not investigated the dietary folate consumption of the participants and could not explain the nutritional folic acid status of their population. To our knowledge, this is the first study to demonstrate the nutritional status and the serum folate level of BTTs simultaneously. The control group included age-matched, sex-matched and BMI-matched individuals from the same household where the cases were recruited. Therefore, the impact of socioeconomic and genetic factors on results was minimal.
Several studies not involving individuals with BTT from Sri Lanka have identified folate deficiency to be common in people of adolescent age groups. They include a study from Southern Sri Lanka where folate deficiency was found in 54.6% of school boys and 52.5% of school girls between the ages of 12 and 16 years.19 Two separate studies found that 43.6% of adolescent girls20 and 28% of school dropouts21 are folate deficient in Colombo (the capital city). The high folate deficiency in the community found in Sri Lanka seems to be no different from studies done in South and Southeast Asia. Two studies of adolescent girls reported that the prevalence of folate deficiency in Karnataka, India,12 and the delta region of Myanmar22 was 19% and 54%, respectively. A similar high prevalence (40–50%) was reported from studies of pregnant women in Southeast Asia23 and 26.3% of pregnant women in a rural area of Haryana, India.24 The high prevalence of folate deficiency is not limited to Asia. 31.3% of Ethiopian women between 15 and 19 years,25 and 20.1% (36 of 179) and 14.7% (61 of 416) of adolescent girls from rural and urban areas of Edirne, Turkey,26 were folate deficient.
In the current study, no significant differences were observed in dietary folate intake between BTTs and controls for any age group. Likewise, total energy and protein intake between cases and controls also showed no differences between the two groups (online supplemental table). This is likely to be influenced by the study design, where controls were recruited from the same households as those with BTT. The low dietary intake of folate and total energy in both cohorts identified in this backdrop cannot be ignored. Similarly, the daily average intake of total protein was less than the recommended dietary allowance for Sri Lankans in most age groups, especially in age groups of 12–15 and 16–18 years.16 The high anaemia prevalence in the control group (18–20% across the age groups studied) is in keeping with anaemia prevalence surveys done in Sri Lanka in children and adolescents.27 The high anaemia prevalence in the control population could be due to iron deficiency and coexisting alpha thalassaemia, which was not assessed in the current study.
In the present study, family income showed a significant association (p<0.05) with haemoglobin level, dietary folate and protein intake of the control group. Nearly half of the study participants fall into the poor socioeconomic category when compared with the national survey of household income.18 Therefore, socioeconomic status and related factors such as nutrition intake and dietary diversification can affect the folate status of people. As in the current study, Htet et al reported poor dietary folate intake in the folate-deficient group of their study.22 According to Obeid et al, dietary folate intake was not sufficient to achieve optimal folate status in the majority of German women who were not exposed to mandatory folate fortification.28
There are very few studies that have assessed the therapeutic benefit of folic acid in those with BTT. In one such study with symptomatic BTT, Tabei et al reported that folic acid supplementation could improve Hb levels of BTTs from 116 to 119 g/L.29 After administration of folic acid for 3 months, bone pain complaints and symptoms of fatigue decreased.
Folic acid replacement is not without its own risks.30 The adverse effects of excessive folic acid intake include exacerbation of the clinical manifestations of vitamin B12 deficiency among elderly people,31 impaired natural killer cell cytotoxicity among women more than 60 years old,32 development of dihydrofolate reductase polymorphism and33 development of prostate cancers.34 Thus, it is clear that arbitrary folic acid prescription for people, including for those with BTT, should not be considered a harmless practice. Though there might be a benefit of folic acid supplementation for those individuals with BTT with deficiency, our study was not designed to assess this. The high prevalence of serum folate deficiency in those with BTT as well as the controls, together with the overall low dietary folate consumption, justifies the recommendation of food fortification with folate.
Clinical values and limitations
To the best of our knowledge, this study represents the most comprehensive analysis to date on the serum and dietary folate of BTTs and healthy controls. We would like to acknowledge several limitations in our study. First, the subjects of this study primarily consisted of participants from a single thalassaemia centre, potentially impacting the results due to geographical location, lifestyle and dietary habits. To strengthen the evidence base, future studies may expand the sample size and include participants from different regions and multiple localities. Not measuring red cell folate and only using serum folate as a marker of folic acid deficiency is a technical limitation. Moreover, the dietary intake of each participant was determined by recording a 24-hour dietary recall. The inherent ‘recall’ losses of this method are acknowledged by us.
Conclusions and recommendations
There were high levels of folate deficiency in both controls and those with BTT (>24% and 34%), but those with BTT were no more likely to be folate deficient than the controls. Folic acid supplementation for all with BTT does not seem rational based on our findings.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the ethics review committee of the Faculty of Medicine, University of Kelaniya (Ref. No: P/209/11/2018). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors would like to thank all participants for their enthusiastic collaboration and the personnel of the Adolescent and Adult Thalassaemia Care Centre, Ragama, Sri Lanka for their support.
References
Footnotes
Contributors AP conceived the research idea and designed the study in collaboration with ST and RS. ST, UPJ and TUH contributed to the acquisition and analysis of the data. AP and RS contributed to the interpretation of the data, and ST, UPJ and TUH drafted the article. All authors critically revised the article, agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final article. AP acted as the guarantor.
Funding This work was supported by the Accelerating Higher Education Expansion and Development (AHEAD) project coordinated by the Ministry of Education & Higher Education and University Grants Commission, Sri Lanka. (AHEAD/PhD/R1/AH/040).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.