Article Text
Abstract
Introduction Palliative care (PC) improves quality of life (QOL). However, PC is currently delivered ‘too little, too late’ in heart failure (HF). Timely interventions to enable and reach patients with HF and their caregivers, with PC (TIER-HF-PC) is a novel, nurse coach-led model of PC that integrates PC into HF care. We will compare the effectiveness of TIER-HF-PC against usual care for improving patient and caregiver health outcomes. We will also evaluate implementation outcomes (such as care experience) of TIER-HF-PC.
Methods and analysis In TIER-HF-PC, patients undergo regular distress screening. The intensity of PC treatments will be tiered based on the severity of problems detected. Minimally, all patients will receive PC education resources. Patients with moderate-intensity needs will receive PC health coaching. Patients with high-intensity needs will receive a PC physician consultation, on top of PC health coaching. Patients in usual care are not screened but can be referred to a PC physician based on cardiologist discretion.
We will recruit 240 English- or Mandarin-speaking patients with HF and up to 240 caregivers from 3 sites across 2 cardiac centres. Patients will be randomised in a 1:1 ratio to TIER-HF-PC or usual care. We will use an intention-to-treat approach for data analysis. Our primary outcome is patient QOL on the Kansas City Cardiomyopathy Questionnaire at 24 weeks. Secondary outcomes include patient healthcare utilisation, caregiver QOL and cost-effectiveness. All participants who received PC treatments will receive a service evaluation survey. Additionally, a sample of these participants and their treating healthcare staff will be purposively recruited for in-depth semistructured interviews on their TIER-HF-PC experience. Interviews will be thematically analysed. We will evaluate protocol fidelity through case notes and study process audits.
Ethics and dissemination This study was approved by the SingHealth Institutional Ethics Review Board—review number: 2024–2213. Results of the study will be disseminated when data analysis is complete.
Trial registration number NCT06244953.
- Patients
- PALLIATIVE CARE
- Heart failure
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Assessment of implementation outcomes, in addition to health outcomes.
Adequately powered study.
Recruitment from more than 1 site, allowing generalisability of results.
Limited to English- and Mandarin-speaking patients, with potential to expand to Asian patients speaking other languages in the future.
Introduction
Heart failure (HF) is a serious, life-threatening condition that threatens the well-being of approximately 26 million people worldwide.1 In Singapore, cardiovascular disease is a common cause of death, accounting for 30.9% of all deaths in 2023.2 The illness trajectory of HF is undulating and unpredictable, with the risk of sudden death increasing exponentially as HF progresses towards its advanced stages.3–5 Patients with advanced HF have high physical and psychoemotional burden, poor quality of life (QOL) and are prone to recurrent hospitalisations, especially at the end of life.6–8 Besides the distress HF places on patients, caregivers also have significant caregiving burden, which increases as HF advances.9
Palliative care (PC) is an approach that improves the QOL of patients and their families who are facing problems associated with life-threatening illness.10 PC interventions for HF in cohort studies and pilot studies have been shown to improve patient-centred outcomes, documentation of preferences and reduce inappropriate healthcare utilisation.11 There are strong recommendations from European, American and Singaporean HF societies for PC to be integrated as early as possible, from the point of diagnosis of symptomatic HF to death and bereavement.12–16 According to the 2014 World Health Assembly mandate, all governments should aim to ‘strengthen PC as a component of comprehensive care throughout the life course’.17 The Singapore Ministry of Health in its 2022 work plan for a healthier Singapore similarly exhorted the healthcare workforce to improve PC provision for all patients, as well as embrace holistic approaches to improve wellness for patients and their caregivers.18
However, despite the strong mandate and need for PC,19 patients with HF receive PC ‘too little, too late’. In Singapore, the National Palliative Care Minimum Data set (MDS) was developed to provide information on the longitudinal trends of the demographic and clinical profile of patients who receive PC.20 In the 2022 MDS report, it was estimated that, for patients with non-cancer diagnoses such as HF, the median time from first specialist PC assessment to death was only 9 days.20
There are multiple reasons why patients receive PC ‘too little, too late’. First, current PC services are heavily dependent on a scarce resource of specialist PC clinicians. PC reviews are comprehensive and holistic, which is often an intensive ‘all-or-nothing’ approach for PC delivery. As a result, current PC services will not be able to scale up quickly enough to support the rapidly growing and changing needs of the HF population.21 22 Second, PC service provision is dependent on cardiologist referring practices. Current cardiology practices for referring to PC differ widely,23 24 and there are no mechanisms in place that systemically support cardiologists to know when the opportune moment would be to refer PC. Third, there currently does not exist a system for regular screening of patients for needs to allow proactive engagement of patients and caregivers prior to crises.25 PC services are often referred to only when symptoms are escalating and uncontrolled, limiting the window of opportunity in which PC can make a significant difference.
Therefore, our team developed a novel model of PC—timely interventions to enable and reach patients with HF and their caregivers, with PC (TIER-HF-PC). TIER-HF-PC is a tiered model of PC, that is led by a nurse coach who has background training in PC, with support from a specialist PC physician. In TIER-HF-PC, patients will undergo regular distress screening. The type and intensity of PC treatments will be subsequently tailored to the type and severity of problems reported. The coach will also engage and empower patients and their caregivers to take a proactive approach to their care.
TIER-HF-PC is a complex intervention26 27 with multiple interacting components (screening for needs, tailoring of treatments and provision of treatments). The incorporation of a screening mechanism to detect concerns of patients with HF reduces dependency on heterogeneous cardiologist referral patterns. At the same time, the allocation of PC resources according to needs allows specialist physician resources to be preserved for those who are in severe distress, without compromising access to lower intensity PC treatments. The use of health coaching as a PC treatment allows patients and caregivers to be actively engaged in self-care as a strategy, rather than healthcare services being deployed reactively during times of crises.
To evaluate TIER-HF-PC, we will conduct a two-armed parallel group, open label, randomised controlled trial. The overall aim of this study is to test the effectiveness and implementation of the interacting components in TIER-HF-PC.28
Our specific aims include evaluating the:
Impact of TIER-HF-PC on patient-reported outcomes.
Impact of TIER-HF-PC on caregiver-reported outcomes.
Care experience in TIER-HF-PC.
Implementation fidelity of TIER-HF-PC.
Impact of TIER-HF-PC on healthcare utilisation and survival.
Cost-effectiveness of TIER-HF-PC.
We hypothesise that TIER-HF-PC will be superior to usual care in improving patient and caregiver QOL. We also hypothesise that patients and caregivers will be satisfied with the interventions in TIER-HF-PC, while recognising that specific process modifications might be needed to improve the implementation of TIER-HF-PC.
Methods
Study design
We will conduct a randomised controlled trial comparing TIER-HF-PC (intervention arm) against usual care (control arm) among 240 patients with HF and up to 240 caregivers.
Study setting
Recruitment will take place at two cardiac centres across 3 sites: The National Heart Centre Singapore (NHCS), a national and regional referral centre for cardiology which has two campuses/recruitment sites (Outram in the central region and Sengkang in the north-east region)29 and the Khoo Teck Puat Hospital (KTPH), a 795-bed general and acute hospital in the northern region of Singapore.30 NHCS manages over 120 000 outpatient consultations each year while KTPH serves over 550 000 people in their region. In NHCS, patients who require PC at the Outram campus are referred to PC services from the National Cancer Centre Singapore (NCCS),31 and patients who require PC at the Sengkang campus are referred to the PC service in the Seng Kang Hospital.32 In KTPH, patients who require PC are referred to the PC department in KTPH.
Study timeline
The study is planned to start on 14 August 2024 and is estimated to complete by 31 October 2027.
Study participants
Inclusion criteria for patients: (1) 21 years or older; (2) able to communicate in English or Chinese; (3) be of stage C or D HF, as defined by American College of Cardiology/American Heart Association classification system; (4) have functional limitation of New York Heart Association (NYHA) functional status of at least 2 or worse; (5) be deemed by their cardiologist’s clinical judgement to have an expected prognosis of at least 6 months survival; (6) have had a HF-related hospitalisation event (eg, symptomatic decompensated HF) within 6 months prior to recruitment; (7) have a phone that allows telecommunication. Criteria (3) to (6) are based on medical records documentation. Cardiologists can use the current literature on prognostication to guide their clinical prognostication.33 34
Exclusion criteria for patients: participants who have (1) cognitive impairment (eg, dementia); (2) severe, untreated, active mental illness (eg, major depressive disorder); (3) ventricular assist device implant; (4) non-reversible hearing or visual loss; (5) active drug abuse; (6) already known to a PC service. Criteria (1) to (6) will be based on medical record documentation.
Inclusion criteria for caregivers: we will recruit the direct and unpaid family caregiver of the patient, who is self-reported by the patient to be the main person to be either responsible for up to 4 hours a day of caregiving tasks and/or decision maker/spokesperson with the medical team.35 36 This caregiver may or may not live in the same residence as the patient. Caregivers must be 21 years or above and be able to communicate in English or Chinese.
Exclusion criteria for caregivers: participants who have self-reported (1) cognitive impairment (eg, dementia); (2) severe, untreated, active mental illness (eg, major depressive disorder); (3) non-reversible hearing or visual loss; (4) active drug abuse; (5) or if they are a domestic helper for the patient.
Recruitment
A research coordinator will screen the clinic lists of participating cardiology clinics to identify potentially eligible patients. Patients can also be referred to the research coordinator from the study team investigators. The research coordinator will then approach these patients during their visits to the study sites to confirm their eligibility and obtain informed consent. Research coordinators will not be involved in their clinical care. Participants can be recruited in patient-caregiver dyads or as patients alone.
Randomisation
Patient participants will be randomised in a 1:1 ratio to either the TIER-HF-PC intervention or usual care. If the patient’s caregiver is also recruited, he/she will follow the patient’s allocation assignment. Patients will be randomised using the permuted block technique, stratified by recruitment site and by whether the patient has a participating caregiver. The randomisation scheme will be generated by an independent statistician, with the block size kept unknown to the clinical investigators as per the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guidelines. A randomiser who does not participate in the recruitment procedures will reveal the allocation status (generated by the statistician) to the research coordinator. Allocation status will only be made known to the research coordinator after informed consent is taken for the participants. Participants will not be blinded to their allocation status.
Usual care
Guided by the National Institutes of Health (NIH) consensus panel recommendations,37 usual care will be the best comparator to test whether patients with HF and their caregivers can be better supported beyond what is currently offered. Patients in usual care will continue to receive clinical care by their cardiologist. If their cardiologist picks up their symptoms or other concerns, they can be referred to a specialist PC physician by the cardiologist.
Timely interventions to enable and reach patients with HF and their caregivers, with PC
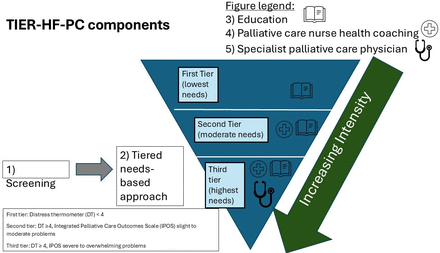
Figure 1 shows the TIER-HF-PC structure. Every 4 weeks, patients will be screened using the distress thermometer (DT).38–40 This screening will last for a total of 24 weeks from baseline recruitment. The screening results will be used to inform the tier of care that they will receive. If a caregiver is recruited together with the patient, the caregiver will be placed in the same tier as the patient.
Components of timely interventions to enable and reach patients with HF and their caregivers, with PC (TIER-HF-PC).
Patients with a DT score ≤3 will be triaged to the first tier (lowest needs). This is consistent with the cut-off score of 4 being the optimal score for screening for patients whose distress level warrants PC.40 Patients who have a DT score ≥4 will be further assessed with the Integrated Palliative Care Outcome Scale (IPOS)41 to allow characterisation of the PC issue. Patients with slight to moderate problems on the IPOS will be triaged to the second (moderate needs) tier, while those with severe to overwhelming problems on the IPOS will be triaged to the third (highest needs) tier. The intensity of PC interventions will be matched accordingly to the tier, with the highest tier requiring the most PC intervention. As screening will occur every 4 weeks, should DT scores increase during the period of screening, patients can be escalated up the tiers accordingly. Patients in TIER-HF-PC will continue to receive clinical care from their cardiologist.
Procedures for the screening process
The DT will be first be sent via text message by the study coordinator. However, if the patient does not respond within the same day, follow-up phone calls for 3 consecutive working days (up to twice a day) will be made by the research coordinator, to ensure we will receive the DT results that are captured in a timely manner. All patients will be contacted by the nurse coach within 3 days of receiving their DT score, so that patients can be further assessed with the IPOS and a timely decision about their tier-placement can be made.
In all three tiers, participants will be given English or Chinese educational resources. This can be given either in hard copy or soft copy, by the research coordinator.
Further details on the tiers follow
First tier (lowest needs): educational resources will contain information on PC services, management of HF symptoms and comorbidities and practical information on caregiving resources in Singapore. These resources have been used in our prior research studies.42–44
Second tier (moderate needs): the health coach will start coaching with patients and their caregivers within 1 week of IPOS screening. Health coaching sessions for patients and caregivers will be conducted separately whenever possible, to ensure patients and caregivers have enough privacy and time to work with the health coach. Depending on the technological literacy and logistical availability of the participants, health coaching can be conducted using videoconferencing methods, over the phone or face-to-face. Each health coaching session is expected to last about 1 hour. We anticipate the health coaching to last over a period of 4–8 weeks. After completion of health coaching, patients and caregivers will receive monthly follow-up calls, up to 24 weeks after baseline, to check in on concerns and reinforce skills taught during coaching. The nurse coach will also facilitate participants to initiate contact back, should concerns arise out of the coaching period. During the coaching period, should a patient have severe or overwhelming concerns, the nurse coach will escalate PC support to the third tier.
Content of health coaching (patient): a structured manual for health coaching will be used. This manual has been prior adapted42 45 46 from the (Educate, Nurture, Advise, Before Life Ends (ENABLE)) programme in the USA.47–53 The ENABLE programme is a nurse-led palliative care and health coaching programme. The culturally adapted ENABLE topics are ‘maintaining positivity and problem solving’, ‘self-care’, ‘coping with stress and spirituality’, ‘symptom management’, ‘talking about what matters most, making choices’ and ‘sharing your journey and legacy’. The sequence of the ENABLE topics will be individualised, based on patient’s requests and results on the IPOS.
Content of health coaching (caregiver): health coaching topics for caregivers are ‘maintaining positivity and problem solving’, ‘self-care’, ‘coping with stress and spirituality’ and ‘being a partner in managing symptoms’.
Third tier (highest needs): patient participants will be reviewed by a specialist PC physician within 1 week of IPOS screening. There will be structured assessments by the physician to evaluate their physical, emotional and social problems, with the specific purpose of allowing medication titration and referrals to other clinical services as necessary. The physician review will be done face-to-face whenever possible. Alternative methods for consulting include video consultation, with the caveat that patients who need physical examination would be called back to the hospital for physical consult, should the physician deem necessary. After completion of the physician review, participants will receive health coaching and follow-up, as per what is done for participants in the second tier.
Participants in all 3 tiers who are still alive after week 48 will be transited by the nurse coach to relevant services in current care. This transition will be determined by their needs and in consultation with the PC physician.
Study instruments and data collection procedures
Collection procedure
All patient-reported and caregiver-reported outcome measures will be collected over the phone, by the study research coordinator. If participants are not contactable over the phone despite attempts, we will collect the outcome measures face-to-face to ensure timely data collection and to minimise missing data.
Study instrument selections
Study instruments were chosen as they have been used in the HF population and in local studies in Singapore in the English and Chinese language.
Study instruments for patients only
Cardiac-specific QOL measure: Kansas City Cardiomyopathy Questionnaire (KCCQ-12),54 55 a 12-item QOL scale, composed of physical function, symptoms, QOL, social interference and a summary score. It is validated in the cardiac population and commonly used in cardiology trials for assessment of QOL. The KCCQ-12 has similar validity to the 23-item version and is shorter with less respondent burden.55 The KCCQ-12 can be self-reported by patients or read to patients over the phone by a research coordinator. The total summary score can range from 0 to 100, with higher scores representing better health status. A clinically meaningful change is defined as a change of at least five points on the KCCQ.56 The KCCQ-12 will be measured every 8 weeks from baseline, till the point of patient death or till week 48, whichever is earlier. The frequency of our proposed KCCQ tracking is referenced from trials which used KCCQ to track longitudinal changes in QOL.56
Non-cardiac specific health-related QOL: European Quality of Life (EuroQoL) EQ-5D-5L57–59 provides a global assessment of the patient’s QOL in five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. It can be administered over the phone.58 Each patient’s health state utility can be derived from their responses to the five dimensions.
Hospital Anxiety and Depression Scale (HADS)60: a 14-item scale with a score range of 0–42, that can be administered over the phone.61 It is composed of two subscales—anxiety and depression—with a cut-off point of 11 for each subscale, that determines anxiety and depression of clinical significance.
Study instruments for caregivers only
Singapore Caregiver QOL Scale −15 (SCQOLS-15): a 15-item scale with five domains measuring QOL of life caregivers in domains: physical well-being, mental well-being, experience and meaning, impact on daily life and financial well-being.62–64 This will be measured every 8 weeks from baseline, till the point of patient death or till week 48, whichever is earlier.
Study instruments for patients and caregivers
Brief Coping with Problems Experienced (Brief-COPE) scale65: a 28-item scale measuring the ways people cope with stressful events. It has been used in the HF population, where the coping style was shown to correlate with the level of physical functioning.66
Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being 12 Item Scale (Facit-SP-12)67: a 12-item survey that measures the spiritual well-being of patients and caregivers. The Facit-SP-12 has been used as a secondary outcome measure in the PC population for HF trials.68 The total of Facit-SP-12 ranges from 0 to 48 with higher scores representing increased spirituality.
Data collection on experience with TIER-HF-PC and its acceptability
Client Satisfaction Questionnaire (CSQ-4)69: a 4-item scale consisting of 3 items (measuring the experience of the client with a programme) and an item measuring improvement in client self-efficacy. This service evaluation questionnaire will be measured for participants randomised to TIER-HF-PC. The CSQ-4 has been used as an outcome measure in our prior studies42–44 and has also been used in other studies to evaluate health services programmes.70 The CSQ-4 can be administered over the phone.
Semistructured interviews: we will purposively recruit patients, caregivers from TIER-HF-PC and healthcare staff whose patients were managed using TIER-HF-PC. To capture diverse views across different demographics, functional status (patients only), background specialty (PC vs HF and physician vs nurse) and years of clinical experience (healthcare staff only), we will use purposive and iterative sampling techniques. An interview guide will be developed based on elements and selected outcomes in Proctor’s taxonomy of implementation outcomes, such as acceptability, appropriateness and timeliness and the reach, effectiveness, adoption, implementation and maintenance (RE-AIM)/Practical, Robust, Implementation and Sustainability Model (PRISM) framework.71 The RE-AIM framework stands for outcomes such as reach, effectiveness, adoption, implementation and maintenance. The PRISM framework is complementary to RE-AIM and is used to identify the multilevel contextual factors that affect the implementation outcomes in RE-AIM.
Data collection for assessment of fidelity to the TIER-HF-PC intervention and study procedures
The fidelity to procedures and extent to which TIER-HF-PC is implemented as planned will follow recommendations from the NIH behaviour change consortium72 and be determined by the following process measures: (1) number of patients who complete the screening DT and IPOS assessments; (2) percentage of patients and/or caregivers who manage to complete health coaching sessions and specialist PC physician review, according to their assigned tier; (3) case note audit and review of clinical entries by TIER-HF-PC staff. All clinical entries by nurse coach and specialist PC doctor that were entered into the electronic healthcare notes from NHCS and KTPH will be audited to review the main themes of PC treatments that were covered during specialist PC review and health coaching. Due to the anticipated high load of clinical entries, we will audit using the techniques of natural language processing (NLP).73 74
Data collection on healthcare utilisation
We will collect data on referral to community PC services and other community support services that patients in both arms might use, such as community nursing services. The date and number of hospital admissions, hospital length of stay for each admission, number of emergency department visits, number and length of intensive care unit admissions and date and location of death will also be collected. We will track if usual care patients are referred to PC consult services.
Data collection on healthcare costs
We will collect data on the cost of hospitalisation bills, emergency department bills and outpatient bills from the hospital record systems. We will also estimate the cost of healthcare provision, based on estimated time staff spend on caring for patients within this trial.
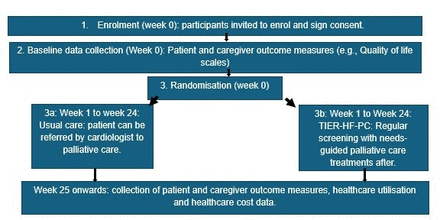
Figure 2 describes a diagram of our study flow and timeline for data collection procedures.
Study flow diagram. TIER-HF-PC, timely interventions to enable and reach patients with HF and their caregivers, with PC.
Statistical justification
Sample size calculation
For the purposes of evaluation of the primary outcome—assuming a common SD of 12 points for the KCCQ overall summary score,68 the planned sample size will be 200 subjects (100 per arm) to provide 80% power at 5% two-sided type 1 error rate, to detect a difference of 5 points at 24 weeks, which is the smallest change that is clinically significant at the individual patient level.51 To account for dropouts and attrition, we will aim to recruit 120 patients per arm (240 patients). As caregiver recruitment is based on the patient sample, we will aim to recruit up to 120 caregivers per arm (up to 240 caregivers). We estimate we will recruit 80% of the participants from NHCS campuses (192 patients and 192 caregivers) and 20% of the participants (48 patients and 48 caregivers) from KTPH. Based on screening logs from previous studies recruiting similar patients from the same settings, it is estimated that there will be 40 eligible patients per month. Assuming a recruitment rate of 50% and 20 patients recruited per month, the sample size could be achieved within 12 months. A more conservative estimation of a 40% recruitment rate (16 patients are recruited per month) would allow us to achieve the required sample size within 15 months. For the semistructured interviews, based on our prior research in the same setting,75–77 we will reach data saturation by 30–40 patients, 30–40 caregivers and 30 staff across two sites. These will be our target sample sizes.
Analysis of patient and caregiver outcome measures
We will adopt the official scoring method to obtain the summary scores and domain scores of the various questionnaires—KCCQ-12, SCQOLS-15, HADS, EQ-5D-5L, Facit-SP-12, subject to imputation for item non-response by the ‘half rule’, if applicable. Descriptive statistics and measures of effect size will be used to compare the study groups at baseline, 8 weeks, 16 weeks and 24 weeks. Analysis will follow an intention-to-treat principle. Longitudinal data analyses will be conducted to examine intervention effects using linear mixed-effects modelling for repeated measures at baseline, 8 through 16 and 24 weeks, constraining the baseline mean to be equal between intervention and control groups with indicators for time, group and time by group interactions. Mixed-effects models using maximum likelihood estimation provide robust estimates despite missing values or early dropouts if the data are missing completely at random or missing at random.78 The estimate of SD of residuals from the mixed-effects model will be used to compute effect sizes (Cohen’s d). We will also chart the trajectories of QOL from baseline till patient death or week 48, whichever is earlier, to evaluate the differences in QOL across time between arms. In addition, we will perform preplanned subgroup analyses to evaluate associations of outcomes with variables including recruitment site, time since diagnoses of HF, NYHA status and number of PC health coaching and physician review sessions received. We will also examine changes in coping style over time, using BRIEF-COPE scores as an outcome.
Assessment of experience with TIER-HF-PC
The total score of the CSQ per participant will be computed. We will analyse patient and caregiver scores separately and will calculate the percentage of participants in TIER-HF-PC who have at least a CSQ score of 12 and above, which indicates good experience with care.
Regarding the analysis of semistructured interviews, we will form a qualitative coding team (consisting of 2 research coordinators, the principal investigator and a co-investigator). All members of the coding team will be required to have training in qualitative analysis. All audio-taped interviews will be transcribed verbatim by our research coordinators, and the accuracy of each transcript will be cross-checked by another research coordinator. Interviews conducted in Chinese will be translated to English prior to analysis by the coordinators. Coding of up to 30% of our interviews will be initially undertaken by both research coordinators to ensure intercoder reliability in accordance with current guidelines.79 Coding will subsequently be done independently by the 2 coordinators once intercoder reliability is established. Interviews will be open coded line by line. Code categories will be developed and iteratively adjusted through discussions. The code categories will be subsequently mapped back to the main domains of the RE-AIM and PRISM framework to identify key multilevel contextual factors influencing the TIER-HF-PC outcomes as well as to assess the adoption, implementation and impact of TIER-HF-PC. Hence, the analysis will involve both inductive and deductive approaches.
Analysis of fidelity to TIER-HF-PC
For the purpose of auditing TIER-HF-PC, we will follow guidance from the literature for auditing using NLP.73 74 A sample of 30% of extracted medical notes will be annotated to highlight keywords/phrases and develop a standard codebook that will delineate the specific components of our PC health coaching and physician treatments. These will include documentation around: self-care, symptoms, psychoemotional concerns, spirituality, decision making, care planning, financial and legacy planning. Regarding the NLP techniques, we will leverage a class of models pretrained on vast electronic health records. Among these are ClinicalBERT,80 BioBERT,81 BioMegatron82 and GatorTron,83 which have been shown to be performant on clinical concept extraction, natural language inference, medical question answering and semantic textual similarity tasks. We propose the use and exploration of these models as feature extractors in conjunction with other traditional machine learning models (ie, tree-based models) for downstream classification of interview notes into concepts relevant to the TIER-HF-PC objectives. The feature extraction step uses the model to transform an input (ie, text) into an array of numbers that hold information about the input’s structure and semantics from medical notes. This approach leverages existing pretrained models to form the corpus necessary for fine-tuning of specific use cases, in this case, the auditing of TIER-HF-PC clinical notes. For the development of the automated audit algorithm, the annotated notes will be split into 80% training and 20% test datasets for the development of the NLP algorithm. Validation will be based on the 20% of data unseen in the training phase. If the machine-annotated notes appear out of context, they will be examined and the code book will be tuned accordingly. We will report on the sensitivity, specificity, area under the receiver operating curve, F1-Score and the area under the precision-recall curve (AUPRC). Once the target performance is achieved, we will run the software to annotate the remaining 70% of the medical notes.
Assessment of the impact of TIER-HF-PC on healthcare utilisation and survival
We will estimate the differences in community hospice usage, number and length of hospital admissions, number of emergency visits, number and length of intensive care visits between TIER-HF-PC participants and those in usual care using generalised linear models, with binomial model for community hospice usage and Poisson model with robust SE for other healthcare utilisation outcomes.
We will quantify the differences in survival between TIER-HF-PC and usual care by Cox model, censoring at week 48 or death, whichever occurs earlier.
Healthcare cost analysis
We will sum up the total cost of inpatient bills and outpatient bills, including emergency visit bills for TIER-HF-PC participants and usual care participants from week 1 to week 24 and quantify the cost differences between both groups by generalised linear model with Gamma model and robust SE. The net cost of TIER-HF-PC will be calculated based on the cost of care provision in TIER-HF-PC minus the cost savings from the reduction in healthcare utilisation. We will also calculate incremental cost-effectiveness ratios to measure the average net cost per quality-adjusted-life-year (QALY) gained for TIER-HF-PC versus usual care participants. The QALY will be calculated by the product of quality-of-life (EQ-5D-5L) and survival.
Fidelity, standardisation and monitoring
The study team at NCCS will remain responsible for keeping track of the overall study recruitment rate, randomising participants and managing data collection, cleaning and analysis. The data monitoring committee will include the study principal investigator, the statistician and the site principal investigators. Access to the data set is governed through a data sharing agreement with all participating investigators in the study team, governed by the study principal investigator, as per institutional rules. The study principal investigator will coordinate study logistics through regular site meetings between NCCS, NHCS and KTPH. These will ensure timely solving of recruitment, retention and other study issues. Health coaches will have mandatory training using current available resources for ENABLE coaching training prior to the start of TIER-HF-PC. They will use checklists to ensure the coaching and assessments are standardised. They will also have monthly meetings with the principal investigator to troubleshoot problems that arise during the process of health coaching and assessment.
Participant safety
Provisions will be made to recruit patient participants after their acute HF exacerbations have been resolved. Participants might be emotional during health coaching and PC physician review. If this occurs, the principal investigator will refer to existing clinical networks for emotional support. Participants will be allowed to withdraw from the programme at any point in time if requested.
Patient and public involvement
We did not involve any patients or members of the public in the design of this trial.
Discussion
We describe a novel tiered model of PC which we aim to evaluate if it could be effective in improving outcomes in patients with HF and that of their caregivers. Besides effectiveness, we also aim to determine if this model of care would be acceptable, cost-effective and can be implemented with fidelity. In this study, we will also be using novel methods of analysis such as NLP to audit TIER-HF-PC.
Empowering patients to self-care through nurse-led early PC47–53 and implementation of regular symptom monitoring in patients with advanced illness have been studied in various studies separately, but there has not been a model of care that has effectively evaluated if patient-reported screening measures can be used to trigger and personalise the type and intensity of PC treatments. We believe that TIER-HF-PC is the first and will bring novel insights into how we can provide PC most sustainably for a rapidly ageing population and workforce. TIER-HF-PC allows us to evaluate if limited specialist resource can be directed at those most in need while ensuring that all patients have access to a patient-determined, appropriate level of PC, delivered through established care escalation pathways and regular screening.
We do anticipate challenges within this study, such as differing levels of technological literacy within our participants; thus, we have allowed for both telehealth and face-to-face methods for conducting the PC treatments in TIER-HF-PC. We also anticipate that there might be difficulty recruiting caregivers face-to-face, as not all caregivers would accompany patients to their outpatient appointments. Therefore, we will contact caregivers via the phone and allow mail-back options for consent. We are also allowing for recruitment from two cardiac centres with access to three campuses, to have a larger population of patients with HF to recruit from and strengthen the overall generalisability of our study to different healthcare settings. To minimise trial dropouts, we will use phone-based collection of outcome measures to reduce trial fatigue.
If TIER-HF-PC is shown to be beneficial, we will iterate and disseminate TIER-HF-PC to other care settings for HF care in the medium term. In the long term, TIER-HF-PC can potentially be expanded to other serious life-limiting illnesses that have limited PC access. These will include non-cancer illnesses such as chronic kidney disease, chronic respiratory disease and chronic neurological disease.
Dissemination of results
Results of this study will be disseminated upon completion of data analysis. This manuscript, or any related manuscript, is not currently under consideration or accepted elsewhere.
Current protocol version
Version 1.0, version date: 25 December 2023. Any protocol modifications will be updated on clinicaltrials.gov.
Ethics and dissemination
This study has been approved by the SingHealth institutional ethics review board—study review number 2024–2213 and covers all participating sites. All participants must sign informed consent (online supplemental material) prior to participation, and privacy of data will be adhered to as per institutional requirements. Results of the study will be disseminated through publications and research conferences when data analysis is complete.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
Our team would like to acknowledge Professor Bakitas M and Associate Professor Dionne-Odom N in their kind guidance and mentorship for this project.
References
Footnotes
Contributors SH-SN was responsible for drafting of the manuscript, design of the study and finalisation of the manuscript. KY and CFL were responsible for conceptualising the study methods and analysis. YBC was responsible for guiding study design and methodology. All authors approved the final version of the manuscript to be published. SH-SN is the guarantor.
Funding This study was funded by the National Medical Research Council Singapore, project ID: MOH-001452-00. This award also provided 0.5 full-time equivalent salary support to the Principal Investigator Neo SH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.