Article Text
Abstract
Introduction There is a higher proportion of African American care partners due to African American older adults having more than twice the prevalence of Alzheimer’s disease and related dementias (ADRD) compared with non-Hispanic white older adults in the USA. African American adult daughters are the largest group of care partners of African American persons living with ADRD. Within this role, they are faced with navigating multiple health-related decisions to optimise the quality of life of their parent with ADRD, which can negatively influence their own quality of life. This study is guided by three conceptual frameworks: National Institute on Aging’s Health Disparities Research Framework, Black Family Socio-Ecological Context Model and Superwoman Schema. The purpose of this study was to develop a structured culturally responsive prototype intervention that will improve the healthcare decision-making self-efficacy and quality of life of African American parent-adult daughter dementia dyads. The prototype intervention will be piloted in a single-group clinical trial to evaluate feasibility, acceptability and preliminary efficacy.
Methods and analysis This study will be conducted in two phases using an explanatory sequential mixed-methods design, wherein qualitative data collection follows quantitative data collection to explain the findings. In the first phase, the quantitative data collection will examine the behavioural, sociocultural and environmental lifecourse influences on healthcare decision-making self-efficacy and the quality of life of 70 African American parent-adult daughter dementia dyads (ie, persons living with ADRD and their adult daughter care partners). The qualitative data collection will consist of a nested sample of 15 dementia dyads and focus on the decision-making processes that affect current and future healthcare use for the parents living with ADRD. Further, this study will explore how these processes influence the quality of life of both members. In phase 2, integrated findings from phase 1 will provide the basis for the development of the prototype intervention using design thinking and intervention mapping with key informants and community advisory board oversight. Once the prototype intervention is developed, a clinical trial will be conducted. This trial will enrol a new sample of 20 African American parent-adult daughter dementia dyads using a pretest–post-test design.
Ethics and dissemination The first phase of the study has been approved by the Emory University Institutional Review Board and is registered on ClinicalTrials.gov (NCT05139290). This study will contribute to the development of a structured culturally responsive prototype intervention for African American parent-adult daughter dementia dyads. The findings will determine whether a larger randomised controlled trial is warranted. Results of the research will be disseminated in both academic and community settings.
Trial registration number NCT05139290.
- Aging
- Dementia
- Decision Making
- Primary Health Care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The structured culturally responsive prototype intervention will be designed using an explanatory sequential mixed-methods approach.
Because the study is entirely remote, we developed protocols to support collecting data via phone or paper instead of videoconferencing.
Using a single-group clinical trial design is a limitation, but we will gain insights about African American parent-adult daughter dementia dyads.
Introduction
African American older adults are suggested to have more than double the prevalence of Alzheimer’s disease and related dementias (ADRD) compared with non-Hispanic white older adults in the USA,1 which increases the burden of ADRD caregiving in this racial group.1 This burden disproportionately falls on African American adult daughters who are typically the care partners of African American persons living with ADRD.1–3 Bonds et al 4 found that African American non-spouse (primarily adult daughter) dementia dyads (ie, African American persons living with ADRD and their African American care partners) both experience worse quality of life than both members of African American spouse care partner dementia dyads. African American adult daughters must navigate caregiving through the lens of culture, race, gender and associated social determinants of health that lead to health disparities5 6 while providing for their own families.7 8 African American adult daughters may benefit from access to structured programmes specifically aimed at guiding African American dementia dyads and their families through healthcare decision-making processes for African American persons living with ADRD.9 10
While there is limited demographic information specific to African American adult daughter care partners, among care partners in the USA, the percentage of African American care partners of older adults has increased from 77% to 88% from 2015 to 2020.11 Of these African American care partners, the majority are female (66%), aged 35–64 years old, more likely to have the older adult living in their home (ie, care partner’s home), have children or grandchildren living with them, and 54% are oftentimes in more ‘complex caregiving situations’ than non-Hispanic white care partners.11 Compared with those in 2015, African American care partners in 2020 are more likely to monitor their care recipient’s health (74% vs 62%), communicate with healthcare professionals (71% vs 56%) and advocate for their care recipient (62% vs 47%).11 All these tasks are important components of healthcare decision-making.
Healthcare decision-making processes for African American persons living with ADRD are shaped by lifecourse influences,5 including behavioural (eg, communication), sociocultural (eg, cultural identity) and environmental (eg, health literacy and patient satisfaction).5 Behaviourally, styles and methods of communication in the healthcare system are often opaque12 and stand in contrast to and at odds with family-based styles and methods commonly desired in African American families.3 13–15 Socioculturally, African American care partners are concerned about healthcare decision-making processes that might produce decisions that do not reflect the African American person living with ADRD’s desires.3 Tensions can arise within dyads and/or families when African American care partners want to make healthcare decisions about services that go against the desires of African American persons living with ADRD.3 African American care partners have reported delaying healthcare decisions as a way to respect the older adult and maintain the family dynamic.16 In broader environmental terms within the USA, African American care partners worry that their healthcare decision-making process for African American persons living with ADRD might be ill-informed (eg, not knowing the best option), either because their own health literacy regarding navigating healthcare services6 17 or because they have unknowingly misplaced trust in the wrong person when making healthcare decisions.3 This concern is further exacerbated for African American care partners by their understandable distrust in the healthcare system due to known differences in their healthcare experiences.17 18 Research suggests that shared decision-making approaches can reduce these dilemmas for care partners.19
In addition to lifecourse influences, family dynamics within African American parent-adult daughter dementia dyads (hereafter referred to as dementia dyads) affect the quality of life of both members of these dyads. For African American care partners specifically, the relationship between care partners and African American persons living with ADRD matters; non-spouse care partners experience significantly worse quality of life.4 In addition, family dynamics influence the quality of life of African American care partners; greater reports of dyadic strain within African American dementia dyads were significantly associated with worse quality of life for African American care partners.4 In a literature review focused on African American women care partners of individuals with chronic illnesses, including ADRD, African American women care partners who reported poor family functioning experienced worse quality of life.8
Involvement of African American persons living with ADRD in daily care decision-making (eg, what to eat, what to wear) significantly contributes to a better quality of life for African American persons living with ADRD.4 20 Yet, to our knowledge, the influence of healthcare decision-making processes within African American dementia dyads on their quality of life has not been examined. In the broader context of healthcare decision-making within dementia dyads, persons living with mild to moderate ADRD desire to be included in healthcare decision-making regarding their care.21 Care partners often overlook this desire and focus on the person living with ADRD’s capacity to participate in the healthcare process.21 Supporting healthcare decision-making for African American persons living with ADRD by meeting the educational and psychosocial needs of African American dementia dyads may hold promise in improving their quality of life.
Structured programmes focused on education and support have positively influenced quality of life.10 22 Specifically, structured programmes have been found to improve the quality of life of both members of dementia dyads.10 In a systematic review of structured intervention programmes for dementia dyads, participation in these programmes was suggested to be particularly beneficial for under-represented groups.22 However, few structured intervention programmes address racial differences in ADRD caregiving, are culturally responsive and include dyadic research.10 23
The proposed study aligns with the recommendation of the National Institute on Aging’s Dementia Care Summit24 to ‘use theory-driven frameworks’ and ‘address the complex heterogeneous and interacting challenges experienced by persons living with ADRD and their caregivers.’ This study is guided by three frameworks: the National Institute on Aging’s Health Disparities Research Framework,5 the Black Family Socio-Ecological Context Model25 and the Superwoman Schema.26 The National Institute on Aging’s Health Disparities Framework5 highlights levels of analysis (ie, behavioural, sociocultural and environmental) relevant to health disparities in African American dyads and families. The Black Family Socio-Ecological Context Model25 provides a conceptual basis for examining societal and lifecourse influences including health disparities within individual, dyadic and family spheres. Finally, the Superwoman Schema26 specifically focuses on the influences of cultural characteristics of African American women, which will be applied to the African American adult daughter care partners (referred to as care partners beyond this point).
Methods and analysis
Study design and study setting
The study is being conducted at Emory University’s Nell Hodgson Woodruff School of Nursing with enrolment occurring across the USA. Recruitment across the USA was chosen purposively to strengthen heterogeneity in the sample. This National Institutes of Health Stage 0-1B study27 will use a mixed-methods explanatory sequential design28 to identify key factors that will lead to the development and piloting of a structured culturally responsive prototype intervention (referred to as prototype intervention beyond this point) for African American dementia dyads and/or families of persons living with ADRD. This explanatory sequential mixed-methods study will first collect and analyse quantitative data from surveys and then collect qualitative data from the semistructured interviews to explain the quantitative findings, which will build on each other.28
The study will be implemented in two phases (figure 1). The first phase or preintervention phase will consist of both quantitative and qualitative data collection and analysis. This phase started in June 2022. The first phase will end with the integration of data that will inform the development of a prototype intervention. We anticipate the integration of data will occur by August 2025. The second phase is divided into two parts—intervention development and piloting of the prototype intervention. The anticipated start date for the intervention development is September 2025. The anticipated start date and end date for the pilot are September 2026 and September 2027, respectively.
Summary diagram of the study design.
Familiarity with technology
We anticipate that technology will be used during all phases of this study. If needed to improve familiarity with the proposed technology, the first author or a member of the research team will schedule a time to orient participants. This orientation will include any of the following: (1) an interactive demonstration of the steps of using REDCap and the Zoom videoconferencing platform and (2) a demonstration of using Zoom as a conference call line for those with telephone access only.
Culturally responsive research
The Center for Health and Healthy Aging, which is housed in the University of Minnesota’s School of Public Health, defines culturally responsive research as research that ‘incorporates the knowledge and unique cultural experiences of individuals, children, families, organizations, and communities served’ and is ‘driven by first-hand knowledge and an understanding of the varied needs of diverse families and communities—their choices, not by cultural stereotypes or generalized assumptions.’29 This study protocol is culturally responsive in two ways. First, key informants are an integral part of the design thinking and intervention mapping approaches for the development of the prototype intervention (described below) in the design thinking and intervention mapping approaches we will use to develop the prototype intervention. Second, the D.E.C.I.D.E. Research Lab, which is led by the first author, has a community advisory board (CAB) (described in greater detail below) associated with it. The CAB shares their lived experiences with the first author during quarterly meetings, which guide all the projects coming from this laboratory.
Key informants
Key informants will be used in phase 2 of this study. Key informants will consist of up to six African American dementia dyads who did not participate in any of these phases of the study. All key informants must meet the above inclusion criteria for the parent living with ADRD and care partner, respectively. In addition, key informants must have experience navigating at least two of the following healthcare decisions: getting a formal diagnosis of ADRD for the parent living with ADRD, finding and/or changing a healthcare provider of the parent living with ADRD, managing the parent living with ADRD’s medications under the guidance of a healthcare provider, deciding the parent living with ADRD will receive a treatment or have a procedure, participating in conversations about hospice, palliative care and/or end-of-life care with a healthcare provider for the parent living with ADRD. Key informants will receive an honorarium for their participation.
Design thinking
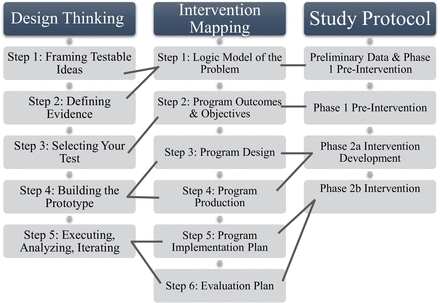
Design thinking is often described in five iterative steps: empathise, define, ideate, prototype and test. We will use the five steps of experimentation based on design thinking approaches.30 These five steps are (1) framing testable ideas; (2) defining evidence; (3) selecting your test; (4) building the prototype; and (5) executing, analysing and iterating.30 The design thinking will be carried out at the Design Studio at Emory University’s Roybal Center for Dementia Caregiving Mastery. The Design Studio uses the design thinking process to challenge and refine an emerging product, which is grounded in the early and frequent involvement of key informants or end users in product design.31 Through a facilitated process by a design thinking specialist of divergent-convergent fast track engagement of appropriate intellectual resources to clarify ideas and design testable user-centred prototypes. The design studio sessions will be conducted in three study visits with the key informants using focus group methods. A member of the research team will attend and document non-verbal cues during each session.
Intervention mapping
Intervention mapping is a six-step iterative process, which consists of developing a logic model of the problem; programme outcomes and objectives (eg, logic model of change), programme design, programme production, programme implementation plan and evaluation plan.32 Intervention mapping sessions will be facilitated by the first author using the Intervention Mapping Workbook and accompanying textbook.32 During this facilitated process, the prototype intervention will be developed, piloted and evaluated.
A total of six sessions (ie, three design thinking sessions and three intervention mapping sessions) are planned to occur over a 6-month period for prototype intervention development. A fourth session of both will occur after piloting to evaluate the prototype intervention. The size of each design thinking session will be limited to 8–10 participants and include key informants and research team members. Intervention mapping sessions will have members of the research team and CAB. All sessions will last approximately 90 min, be audio-recorded and transcribed verbatim. Refer to figure 2 for how these methods will align with the study design.
Alignment of design thinking and intervention mapping with prototype intervention.
Patient and public involvement
Community advisory board
The CAB currently consists of non-participant healthcare providers and staff, African American adult daughters, African American older adults and African American persons living with cognitive impairment. The CAB was established in 2022 and is affiliated with the first author’s laboratory. The research questions and outcome measures were determined prior to establishing the CAB. However, CAB members acknowledge the importance of the research question and outcome measures. The CAB members provided suggestions and insights on how to conduct the study visits with the parent-adult daughter dyads often based on their own lived experiences. The CAB members are involved in developing and disseminating recruitment materials, determining the compensation of participants and identifying inclusion and exclusion criteria. The CAB will actively participate in data analysis and interpretation of the findings to develop the prototype intervention. Specifically, CAB members will provide feedback on data collected in phase 1 (the preintervention phase) and phase 2 (intervention development and intervention). The results of the study will be presented to the CAB members during one of our quarterly meetings as well as sent to them via email. The authors will disseminate the findings via publications, conference presentations and community events. Community events are often recommended by CAB members. Each member of the CAB is given an honorarium for each meeting they attend.
Phase 1: preintervention
Inclusion and exclusion criteria of parent-adult daughter dyads
Inclusion and exclusion criteria for the study participants are provided below. Inclusion criteria for the parent living with ADRD consists of the following: (1) self identifies as African American; (2) age 50 years or older; (3) community dwelling; (4) experiences signs and symptoms of mild to moderate dementia through family care partner’s report on the Dementia Severity Rating Scale;33 (5) able to read, speak and understand English; and (6) willing to participate. Exclusion criteria for the parent living with ADRD (referred to as parent hereafter) are any confounding significant neurological diseases (eg, Parkinson’s disease) or a major psychiatric disorder (eg, schizophrenia).
Inclusion criteria for care partners (referred to as daughter hereafter) consist of the following: (1) self identifies as African American; (2) at least 18 years of age; (3) daughter or daughter-in-law (including fictive kin individuals); (4) aids in activities of daily living and/or instrumental activities of daily living for their parent; (5) makes/takes part in healthcare decisions; (6) able to read, speak and understand English; (7) cognitively intact; (8) access to an internet connection for hosting videoconferencing; and (9) access to a phone or computer that accepts emails.
Scheduling and consent process
Interested participants are guided to the study’s REDCap prescreening survey by an Institutional Review Borad (IRB) study flyer or after contact with the research team. Completion of the prescreening survey takes approximately 10 min. Once the prescreening survey is completed, the research team is notified and contacts the potential participant within 3–5 business days via email or phone based on the potential participant’s preference. After this initial contact is made, all interested, eligible participants must participate in a scheduling call. The scheduling call was implemented for four reasons: (1) to schedule the study visit, (2) to begin to build rapport with the daughter, (3) to answer study questions and (4) to screen for fraudulent participants. The scheduling call has become an important element in the recruitment process because the daughters learn more about the study, get to speak to the first author directly and can provide additional information to help the study visit run smoothly for the parent.
The study visit is scheduled for 2.5 hours, and participants are instructed to have their camera on for the duration of the visit. This time was determined after the first few study visits lasted at least this long due to lengthy conversations with the participants and/or technical concerns. A waiver of documentation of consent was applied to this study. Verbal consent is obtained at the beginning of the study visit. To determine if the parent can provide consent, the parent answers six questions from the University of California, San Diego Brief Assessment of Capacity to Consent tool.34 Parents must answer at least four questions correctly to be able to independently consent. Otherwise, their daughter or other family member provides verbal consent, and the parent provides verbal assent.
Quantitative data collection
The quantitative aim of this study is to identify behavioural, sociocultural and environmental influences associated with the healthcare decision-making processes and quality of life of African American dementia dyads. Primary data collection is through online surveys using REDCap hosted at Emory University (UL1 TR000424).35 Refer to table 1 for a list of surveys. Data collection will take about 30–40 min for the parents and 40–50 min for the daughters. During each study visit, the parent is questioned first to minimise fatigue. The first author reads the questions and marks the answers using the paper-pencil method. The answer choice options for each survey are displayed on the shared videoconferencing screen, so the choices remain visible for the parent. After the parent completes their portion of the surveys, the first author emails the REDCap survey to the daughter to complete during the allotted study visit or at their earliest convenience. Each member of the dyad will be compensated with a $25 gift card for their time (total $50 per dyad). Dyad members choose between three retail gift card options.
List of measures used in screening and preintervention phase
Sample size estimation
Based on G*Power36 analysis to conduct a multiple linear regression with a two-sided α=0.05, 80% power and six predictors, a projected sample size of 60 dyads is needed to detect a moderate-to-large effect (Cohen’s f2=0.26). The rule of 10 participants (ie, dyads) per degree of freedom has been applied based on a similar sample size used in previous dyadic research.4 37 Our proposed sample size of 70 African American dementia dyads will allow for a 10% attrition rate of participants or missing data.
Quantitative data analysis
Preliminary bivariate analysis will be conducted in Stata IC38 to determine predictors of quality of life and healthcare decision self-efficacy from the data. No more than six predictors will be used in linear multiple regression models. Multilevel modelling, specifically Actor-Partner Interdependence Modelling,39 40 will be conducted in HLM software.41 If the sample size is less than our anticipated goal, we analyse parents and daughters separately using within-group effect sizes. Descriptive statistics will be computed through Stata IC to examine sample characteristics. Quantitative data analysis will be completed before qualitative data collection. We will match the domains of the surveys that are statistically significant to the interview questions for qualitative data collection.
Qualitative data collection
The qualitative aim of the study is to describe the decision-making processes of members of African American dementia dyads. After quantitative data analysis, we will connect the sample by choosing a subsample of the dyads to complete the qualitative interviews using maximum variation sampling based on diverse neighbourhoods (eg, rural, urban, suburban), relationship of parent (eg, mother, father, in-law) and acute care visits of the parent living with dementia (eg, none vs one or more).
Each qualitative interview visit will be scheduled to last 1 hour. The visit will be conducted virtually using Zoom. The first author or a member of the research team will conduct the qualitative interviews using a semistructured interview guide. Both members of the African American dementia dyads will be interviewed together (about 30 min). Then, each member of the dyad will be interviewed separately (about 15 min for each),42 with the interviews of the parent occurring before the daughters to prevent fatigue of the parent. This process was successful in the first author’s preliminary research study.43
There are two reasons for dyadic interviewing.44 First, having the participants together initially will help with building rapport, assuage possible apprehension with the interview process and allow for the first author to observe the interactions between the dyad members. Second, separating the dyads will allow for more in-depth interviewing to enhance trustworthiness, validity and comparison and contrast. Individual interviews also allow either a member of the dyad to speak more freely and confidentially about their role or the other member of the dyads’ role in decision-making.
Participants will be notified of the recording of interviews before consenting to the study and will be reminded prior to the recording at the start of the semistructured interview. Audio/video recordings will be generated via Zoom. Recordings of the semistructured interviews will be transcribed verbatim. Following each interview, the first author will create field notes and conduct reflexive journaling during this portion of the study.45 46 Sampling will continue until saturation (ie, no new information or themes emerge from the data) is achieved.47 Each member of the dementia dyad will receive a $25 gift card after completing the semistructured interview as compensation for their time (totaling $50 per dyad). There is potential for up to two follow-up visits to clarify questions and for member checking the themes generated from the data for reliability and validity of the results. Each follow-up interview will last no more than 1 hour. No other information will be collected from the participants during the follow-up visits.
Qualitative data analysis
Using deidentified data, all transcripts will be managed in MAXQDA software.48 The transcripts will be analysed using directed qualitative content analysis to provide a more structured approach to identifying relationships among variables,49 which will lead to the identification of key components of the healthcare decision-making processes from the perspectives of the dyads. The research team will independently review transcripts and identify codes. Other members of the research team who are not a part of the initial coding of the data will be brought in to resolve any discrepancies among the independently developed codes. Member checks will occur to serve as validation of the interpretation (if the participants agree to future contact by the research team).
Integration of preintervention data
The integration of the phase 1 preintervention data will allow the explanation of the quantitative survey items with the qualitative results, themes and quotes, through use of a joint display. Following steps 1–3 of design thinking and steps 1–2 of intervention mapping, this phase will end with the prototype intervention programme outcomes and objectives.
Phase 2: development of prototype intervention and piloting
Phase 2a: intervention development
The second phase of this study begins with the development of a prototype intervention (2a). Integrated findings from the phase 1 preintervention phase of this study will be used to develop the prototype intervention following step 4 of design thinking and steps 3–4 of intervention mapping. An outline for the prototype intervention will be developed. This outline will consist of the prototype intervention programme plan (eg, themes, scope and mechanisms of action), programme materials and messaging, and programme use (eg, adoption, implementation and maintenance). The outline will serve as the basis for course design activities with the Roybal Center instructional consultants and specialists. The outline will be used to create mock-ups of the curriculum and materials. These mock-ups will be shared with the key informants through an iterative process. This process will capture the feedback of the key informants, integrate the changes and result in the presentation of new mockups. Other prototype intervention specifics (eg, number of sessions, which member of the dementia dyad will participate in these sessions, psychoeducational vs counselling, synchronous vs asynchronous, in-person vs online) will be determined during these sessions. Once the key informants, CAB and research team come to a consensus, the prototype intervention will be finalised, and then the first author will start a second recruitment.
Phase 2b: intervention
Implementation and evaluation
Phase 2 will end with the implementation and evaluation of the prototype intervention (2b). The implementation of the prototype intervention will consist of 20 African American dementia dyads who have not previously participated in this study. A $25 gift card will be given to each member of the dementia dyad at each data collection time point and after completing the semistructured interview as compensation for their time (totaling $200 per dementia dyad).
Eligible dyads will be scheduled for one baseline study visit and three follow-up visits post intervention (refer to figure 3). The three follow-up visits will consist of a 1-month study visit and two 3-month study visits. Quantitative data collection will be collected at baseline and the two follow-ups post intervention. The parents will complete the Montreal Cognitive Assessment at baseline only. The Quality of Life-Alzheimer’s Disease Scale and Decision Self-Efficacy Scale will be completed by both members of the dyad at baseline (eg, pretest) and at the two follow-up visits (eg, post-test) to examine changes in quality of life and healthcare decision self-efficacy. Given our current success with dyadic data collection, we anticipate baseline and follow-up visits should last no more than 30 min for the parents and 40 min for the daughters, except the follow-up visit part two at 3 months post intervention.
Timeline for intervention implementation and evaluation.
Part two of the 3-month follow-up visit will consist of a qualitative interview that will last about 45 min, which will occur within 2 weeks of the quantitative 3-month follow-up part one. The dementia dyads will first be interviewed together and then separately. The interviews held with both members will focus on their perceptions of the prototype intervention. The individual interviews will allow for feedback about the prototype intervention but will focus on their perceptions of their quality of life and decision self-efficacy. Questions on the interview guide will focus on the usability and acceptability of the prototype intervention to help with future refinements. The first author, in collaboration with the CAB and research team, will develop an interview guide to conduct the qualitative interviews. Interviews will be recorded and transcribed verbatim.
Recruitment and consenting process
The recruitment, consent and scheduling processes that were used in the preintervention process will be the same for the piloting of the prototype intervention with one exception. The 20 dementia dyads piloting the prototype intervention will not have participated in the preintervention phase of this study.
Sample size estimation
The rule of 10 participants (ie, dyads) per degree of freedom will be applied. We conducted a power calculation using G*Power to provide a comparison. If we conduct repeated measures analysis of variance with a two-sided α=0.05, 80% power and two predictors, a projected sample size of 15 dementia dyads is needed to detect a large effect (Cohen’s f2=0.39). We will recruit 20 dementia dyads to control for a potential 20% attrition rate.
Components of the intervention
Since the prototype intervention will be created in this study, we cannot describe the prototype intervention. We anticipate the prototype intervention will be family-based with a focus on issues of communication, problem-solving, health system literacy and family systems. Once developed and evaluated, the intervention protocol will be published.
Study outcome measures
Primary outcomes
The primary outcomes of this study are feasibility, acceptability, usability and preliminary efficacy. Feasibility will be measured based on the ability to recruit and retain the targeted number of participants within the desired timeframe with full participation. The recruitment plan is for 20 African American dementia dyads to be enrolled in ≤6 months. The actual number of dyads recruited will be reported as the outcome with the percentage of the goal achieved (actual/goal). Feasibility will assess the ability to recruit 60% of the sample. Acceptability will be measured based on the ability to retain participants in the intervention and in the study. Attendance of participants will be monitored during the programme sessions of the prototype intervention. Retention will assess the ability to maintain 70% attendance in intervention programme sessions and 80% of participants from baseline to 3-month follow-up part two. Usability will focus on the ease of use and desire to use the prototype intervention from the perceptions of the dementia dyads. Acceptability will assess the appropriateness of the prototype intervention as well as satisfaction with how it was delivered from the perspectives of the dementia dyads. Data about usability will be gathered during the qualitative interviews at the 3-month follow-up visit part two.
Preliminary efficacy will be determined by the change in the quality of life of both members of these dyads and the change in decision self-efficacy for the adult daughter of the parent. Using the Quality of Life-Alzheimer’s Disease Scale, we anticipate that the change from baseline to follow-up in quality of life will be significantly better (p=0.05) or trend towards significance for both parents living with ADRD and adult daughters. To evaluate self-efficacy with healthcare decision-making, we will use the Decision Self-Efficacy Scale. We anticipate a significant (p=0.05) positive change in both the parents and their adult daughters or a trend towards significance. Effect size will be examined for both analyses.
Data analysis
To evaluate the feasibility, acceptability, usability and preliminary efficacy of the prototype intervention, we will use quantitative, qualitative and mixed methods. Feasibility will be determined by using descriptive statistics. Usability and acceptability will be analysed using directed content analysis.49 Qualitative data from the interviews will be managed in MAXQDA software.48 For the pretest–post-test preliminary efficacy analysis, longitudinal dyadic analysis will be conducted in Stata IC.50 51 Descriptive statistics will be computed for measures at each time point. Internal consistency reliability will be assessed for each measure by computing Cronbach’s alpha for complete item responses. You could look at parents and daughters separately using within-group effect sizes or how you described it above, but I would do some combination of individual within change for daughters and parents and then some way of examining dyads where both benefitted. Given the small sample size, we will conduct within-group effect sizes for the parents and adult daughters separately. There are also three descriptive approaches we can use to evaluate the dyadic benefits of the pilot—above-zero, pretest risk status and/or pattern analysis.52 We will evaluate the data using the above-zero approach. The data from the parent-adult daughter dyads will be coded on each outcome separately based on whether both the parent and adult daughter reported any post-test improvement (ie, above-zero change).52 Qualitative data from the interviews will be embedded in the quantitative data from the preliminary efficacy analysis. The goal of this mixed-methods integration is to refine the prototype intervention based on the piloting.
Discussion
This study examines the feasibility, usability, acceptability and preliminary efficacy of a culturally responsive healthcare decision-making prototype intervention for African American parent-adult daughter dementia dyads. We will examine the potential influence of this prototype intervention on the quality of life of both members of these African American dementia dyads and the healthcare decision-making self-efficacy of African American parents using an explanatory sequential mixed-methods design. The findings from this study will produce a more refined version of this prototype intervention, which will be implemented in a randomised controlled trial.
However, there are some anticipated challenges in conducting this study. First, recruitment may take longer than expected. Recruitment strategies will be discussed during research team meetings to determine the next steps and appropriate amendments to the recruitment plan. Second, there is the potential for incomplete data. Data missing at random will be handled with full information maximum likelihood. Lastly, given the small sample size of this study, non-significant findings with variables of interest are possible. We will look for trends towards significance, calculate effect sizes and incorporate descriptive analysis approaches.
Despite these challenges, the design of this study has many strengths. First, we will identify target(s) of the prototype intervention. These targets will be determined by dyadic and mixed methods analysis. Using multilinear regression modelling, specifically Actor-Partner Interdependence Modelling,53 we will identify quantitative influences at both the individual and dyadic levels. Significant quantitative influences will be matched to interview questions. Second, the inclusion of the perspectives of both members of African American dementia dyads is another strength. The expected outcome is to gain information about the individual, dyadic and family dynamics that influence quality of life and healthcare decision-making of African American dementia dyads.
Ethics and dissemination
Ethical considerations
The first phase of the study was approved by the Emory University IRB. Once qualitative data collection is completed, a modification will be submitted to the IRB to obtain approval for the second phase of the study. The study is registered on ClinicalTrials.gov (NCT05139290).
Informed consent
Participant interviews will be completed only after obtaining informed consent. If assent is obtained from the parent living with ADRD, consent will be obtained from their proxy. Confidentiality of participants’ data will be maintained during the study.
Dissemination
Results will be published in peer-reviewed journals, clinicaltrials.gov and scientific conferences. Community dissemination will include articles in community newspapers and/or newsletters, community forums and health fairs, and other ways identified by the CAB.
Ethics statements
Patient consent for publication
References
Footnotes
X @DrBondsJohnson
Contributors KBJ, KSL, WP and KH contributed to conception and study design. KBJ prepared the first draft of the study design proposal. KSL, WP and KH were involved in critical revisions of the study design proposal. All authors contributed to the finalised study proposal. All authors contributed to the preparation of the manuscript submission. All authors reviewed the manuscript and approved for publication. We want to thank the participants who generously donated their time and openly shared their experiences. We would also like to thank the community advisory board members for their continued support and guidance with this project. KBJ acted as the guarantor.
Funding This work was supported by the National Institutes of Health/National Institute on Aging grant number [K23AG073516] and [K07AG076616] for Dr. Bonds Johnson. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed in written training materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. government.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.