Article Text
Abstract
Introduction Vasopressor support is often preferred as an efficient and convenient way to raise the blood pressure during surgery and intensive care therapy. However, the optimal vasopressor for ensuring organ blood flow and tissue oxygen delivery during surgery remains undetermined. This study aims to assess the impact of norepinephrine versus phenylephrine on cerebral and non-cerebral organ perfusion and oxygenation during anaesthesia in neurosurgical patients with brain tumours. The study also explores the impact of the vasopressor agents on the distribution of cardiac output between various organs.
Methods and analysis This is an investigator-initiated, double-blinded, randomised clinical trial including 32 patients scheduled for supratentorial brain tumour surgery. The patients are randomised to receive a phenylephrine or norepinephrine infusion during preoperative positron emission tomography (PET) examinations and the following neurosurgical procedure. PET measurements of blood flow and oxygen metabolism in the brain and other organs are performed on the awake subject during anaesthesia, following a 10% and 20% gradual increase in blood pressure from the baseline value. The primary endpoint is the between-group difference in cerebral blood flow. Secondary endpoints include detection of ischaemic brain lesions possibly associated with vasopressor treatment, changes in cerebral oxygen metabolism, non-cerebral organ blood flow and oxygen metabolism, cardiac output, regional cerebral oxygen saturation, autoregulation and distribution of cardiac output between organs.
Ethics and dissemination This study was approved by the Danish National Medical Ethics Committee (20 May 2022; 2203674). Results will be disseminated via peer-reviewed publication and presentation at international conferences.
Trial registration number EudraCT no: 2021-006168-26. ClinicalTrials.gov: NCT06083948.
- ANAESTHETICS
- Blood Pressure
- NEUROSURGERY
- VASCULAR MEDICINE
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study’s strength lies in its randomised controlled design, which supports reliable comparisons between the effects of norepinephrine and phenylephrine.
The infusion of commonly used vasopressor agents and clinically relevant dosage regimes ensures the applicability of the study’s results to clinical practice.
The study employs positron emission tomography (PET) technology allowing for multiorgan assessments of blood flow and oxygen metabolism, with PET generally regarded as the ‘gold standard’ for these measurements.
The sequential measurement of cardiac output indices under varying clinical conditions provides the possibility for a comprehensive and currently unknown understanding of vasopressor impact on flow distribution between organs.
The study’s small sample size precludes postoperative clinical outcome assessments of the intervention, while its design prevents intraoperative assessments of organ blood flow and metabolism.
Introduction
Preventing hypotension and maintaining or restoring the patient’s haemodynamic stability during surgery and acute care are fundamental aspects of anaesthetic practice. Substantial evidence associates perioperative hypotension with cerebral and non-cerebral organ ischaemia and dysfunction.1–6 Vasopressor support is often preferred as an efficient and convenient way to raise the blood pressure during surgery and intensive care therapy.7–11 The ultimate goal of the intervention is to restore/maintain blood flow to meet the metabolic demand of the brain and other organs.8 11 12 However, the use of vasopressors per se may also be associated with organ injury, and evidence suggests that the pharmacological profile of the vasopressor may be linked with tissue injury.13 14 The most commonly used vasopressor agents (such as phenylephrine and norepinephrine) have different pharmacological profiles. Phenylephrine, a pure α-adrenergic agonist, primarily acts on the peripheral vasculature. In contrast, norepinephrine engages both α-adrenoceptors and β-adrenoceptors and acts on both the heart and the peripheral vasculature.8 11 Moreover, norepinephrine is a natural catecholamine and neurotransmitter existing in human bodies, while phenylephrine is a synthetic compound. The optimal vasopressor for ensuring organ blood flow and tissue oxygen delivery during surgery and intensive care remains undetermined.14 15
In routine clinical practice, anaesthesiologists and critical care physicians adjust their vasopressor therapy according to blood pressure.11 14 The measured blood pressure only reflects the pressure in larger arteries and not organ perfusion and oxygen delivery to parenchymal cells.14 Thus, blood pressure acts as a surrogate for organ perfusion with varying accuracies. Since organ blood flow and tissue oxygenation are not routinely measured, the treating physician often remains unaware whether their vasopressor therapy achieves the primary goals of maintaining adequate tissue perfusion and oxygen delivery. Evidence from our research group and others suggests that vasopressor agents may be associated with reduced microcirculatory brain perfusion and impaired tissue oxygen delivery during anaesthesia, despite reaching recommended blood pressure targets.9 10 16–19 The data further indicate that cerebral macrocirculation, microcirculation, tissue oxygen delivery and cardiac output (CO) are greater with an indirectly acting α-adrenergic and β-adrenergic agonist (ephedrine) compared with phenylephrine, as observed during anaesthesia in neurosurgical patients.16–19 Collectively, the emerging evidence suggests that vasopressor agents acting on both α-adrenoceptors and β-adrenoceptors, such as norepinephrine, may have the potential to improve blood flow to the brain, organ blood flow and tissue oxygen delivery in anaesthetised patients compared with pure α-adrenergic agonists.
The impact of vasopressors on brain and organ circulation primarily depends on their influence on CO and systemic vascular resistance.8 Physiologically, CO is distributed to different organs and each organ receives its share based on its metabolic demand and organ-specific perfusion-regulatory schemes.20–22 Currently, no data are available on the influence of different vasopressor agents on the distribution of CO (ie, blood flow) to the brain and other organs, such as the myocardium, lungs, kidneys and muscle tissue, during anaesthesia. Theoretically, changes in CO would likely affect blood flow to different organs, including the brain.21 22
The research questions of this study pertain to the potential advantages of administering a combined α-adrenoceptor and β-adrenoceptor agonist during anaesthesia, specifically in terms of its association with improved CBF and tissue oxygenation. Additionally, the study seeks to explore whether this compound offers enhanced blood flow and tissue oxygenation in other organs that are sensitive to changes in blood pressure during anaesthesia, in comparison to the effects of a pure α-adrenergic agonist.
Hypothesis
Our hypothesis is twofold:
Norepinephrine increases perfusion and oxygenation in diseased and healthy brain regions during anaesthesia in comparison to phenylephrine.
Norepinephrine is associated with increased non-cerebral organ perfusion and tissue oxygenation during anaesthesia when compared with phenylephrine.
Objectives
The primary objective is to conduct a comparative analysis of the effects of two commonly used vasopressor agents (phenylephrine vs norepinephrine) on cerebral circulation and brain tissue oxygenation in anaesthetised patients with brain pathology.
The secondary objectives are: (1) to assess and compare the effects of the two vasopressors on blood flow and tissue oxygenation in other organs that are particularly sensitive to changes in blood pressure during anaesthesia. These organs include the heart, lungs, kidneys and muscle tissue; (2) to assess and compare how the two vasopressors influence the distribution of CO between the brain and the organs mentioned above during anaesthesia and (3) to assess and compare the relationship between mean arterial pressure and CBF based on correlation plot and cubic penalised regression splines (ie, static cerebral autoregulation) between norepinephrine and phenylephrine and between normal and diseased brain regions (in addition to the global CBF).
Methods and analysis
Study design and setting
The study is an investigator-initiated, single centre, double-blinded randomised clinical trial. The setting for the trial is the Departments of Anaesthesia, Neurosurgery and Nuclear Medicine and PET Centre, Aarhus University Hospital, Aarhus, Denmark. Patient recruitment began on 1 November 2023, and the anticipated ending date for the study is 31 December 2025. The paper is written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT). See online supplemental material for the SPIRIT checklist and figure. Trial registration: ClinicalTrials.gov NCT06083948.
Supplemental material
Patients
The study will include 32 patients diagnosed with supratentorial brain tumours. Written informed consent (online supplemental file 1) will be obtained by the primary investigator or one of the co-investigators. Adult patients diagnosed with brain tumours will be screened at the outpatient clinic by attending physicians. The inclusion criteria are as follows: aged 18–75 years; scheduled for elective craniotomy for supratentorial malignant and non-malignant tumours with a minimum size of 3 cm (measured as the largest diameter in any plane on MRI); American Society of Anaesthesiologists physical status I–III.16 17 Exclusion criteria include: a history of allergy or intolerance to one of the study medications; active treatment with monoamine oxidase inhibitors; pregnancy (positive pregnancy urine test) or breastfeeding; inability to give written informed consent (online supplemental file 1).
Supplemental material
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research
Randomisation and blinding
Figure 1 shows study design and randomisation. Randomising and blinding are performed by a dedicated nurse or a physician not involved in the experimental part of the study.16 17 Patients and researchers are blinded to randomisation. Patients are randomised in a 1:1 ratio using the in-built randomisation module within the Research Electronic Data Capture (REDCap) system and a randomly varying block randomisation size of 4 and 6 to receive infusion of either norepinephrine (10 mcg/mL) or phenylephrine (100 mcg/mL). The concentrations of study drugs are similar to the concentrations used in previous studies and according to institutional guidelines for the use of intraoperative vasopressor support at Aarhus University Hospital. Identical syringes of 50 mL, containing either norepinephrine (10 mcg/mL) or phenylephrine (100 mcg/mL), are marked with a randomisation code known only to the unblinded nurse or doctor. The study drugs are prepared immediately before administration by a nurse not involved in the actual study. The unblinded persons are responsible for documentation of study drugs, randomisation code, patient ID and blinding of drugs in a specific log. This log will only be accessible to the unblinded person. Code-break of randomisation can only occur in emergencies with suspected adverse patient reaction to a study drug. The staff involved in the experimental part of the study are unaware of randomisation. The randomisation code is kept without the reach of sponsor-investigator. Interpretation of imaging and calculation of flow and oxygenation parameters are performed by a blinded researcher. After the final inclusion, an independent researcher will unblind the two groups and label the groups 0 and 1. Statistical analyses will then be performed, and final unblinding will take place.
Study design and randomisation.
Anaesthesia and monitoring
The anaesthetic management is standardised. General anaesthesia with propofol and remifentanil is administered according to institutional guidelines and titrated to achieve a bispectral index (BIS) score between 40 and 60 which is indicative of an adequate level of anaesthesia for surgery.16 17 A low dose of muscle relaxant (suxamethonium or rocuronium) is administered to facilitate intubation. The patients are ventilated with 40–50% oxygen in air by controlled ventilation, titrated to achieve PaCO2 between 35 mm Hg and 40 mm Hg (4.7–6.0 kPa) and PaO2 >100 mm Hg (13.3 kPa).16 17 Ventilation is adjusted according to arterial blood gas measurements. Isotonic NaCl is infused at a rate of 2–3 mL/kg/hour. Temperature, ECG, oxygen saturation, heart rate, intra-arterial blood pressure and CO indices acquired using LiDCO (Masimo, Irvine, CA) are continuously monitored. The depth of anaesthesia is continuously measured with BIS (Medtronic, MN). Brain tissue oxygen saturation is continuously measured with near-infrared spectroscopy (Medtronic, MN) to allow comparison with brain oxygenation measurements determined with positron emission tomography (PET).16 17
Experimental protocol and intervention
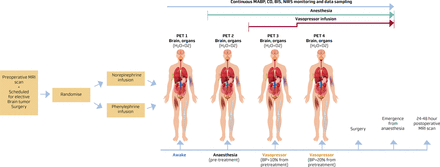
The experimental study protocol and flow chart are shown in figure 2. The experiment is conducted on the same day as the scheduled brain tumour surgery.16 17 All PET examinations consist of a blood flow (using [15O]H2O tracer) measurement followed by a measurement of oxygen consumption (using [15O]O2 tracer).16 Four PET examinations (including blood flow and oxygen consumption measurements) are performed.
Experimental protocol and study flow chart. PET examinations of blood flow (using [15O] H2O tracer) and oxygen consumption (using [15O]O2 tracer) in brain and organs (including myocardium, kidney, lungs and limb muscle) are performed using a Biograph Vision Quadra-PET scanner with a wide axial field of view. The wide axial field of view allows for simultaneous assessments of the brain and organs. The first PET examination (PET 1) is performed in the awake patient. The patient is then anaesthetised, and the PET examination is repeated (PET 2). Vasopressor infusion is initiated and titrated to a 10% increase in MABP relative to pretreatment level (PET 3). MABP is further increased to 20% relative to the pretreatment level (PET 4). After completion of the PET examinations, the anaesthetised patient is transported to the surgical theatre and surgery is performed. The vasopressor infusion is initiated after PET 2 and terminated at the emergence of anaesthesia after completion of surgery. MABP, CO, BIS and NIRS are measured during PET examinations and surgery. The duration of the PET part of the study until initiation of surgery is approximately 2 hours. The 24–48 MRI examination is performed as a postoperative control of the surgical result and includes a diffusion-weighted sequence to detect ischaemic brain lesions possibly associated with vasopressor infusion. BIS, bispectral index; BP, blood pressure; CO, cardiac output; MABP, mean arterial blood pressure; NIRS, near infrared spectroscopy; PET, positron emission tomography.
The first PET examination (PET 1) is performed on the awake patient. The patient is then anaesthetised, and the PET examination is repeated when the anaesthetic depth corresponds to a BIS value between 40 and 60. To avoid significant hypotension after anaesthesia induction, a carefully balanced anaesthesia induction is used, including administration of a crystalloid fluid bolus and, if necessary, atropine (PET 2). Vasopressor infusion is initiated and titrated to increase mean arterial blood pressure (MABP) to above 60 mm Hg or by 10% relative to baseline. When the blood pressure has stabilised the third PET examination (PET 3) is performed. After completion of PET 3, MABP is further increased to above 70 mm Hg or by 20% relative to the baseline level. When the blood pressure has stabilised the fourth PET examination (PET 4) is performed. After completion of the PET examinations, the anaesthetised patient is transported to the surgical theatre and surgery is initiated. During the surgical procedure, MABP is maintained between 70 mm Hg and 80 mm Hg according to institutional guidelines. The vasopressor infusion is terminated at the time of extubation. The duration of the PET part of the study until initiation of surgery is approximately 2 hours. The 24–48 hour postoperative MRI examination is conducted to assess the result of the surgical intervention and to determine whether there are any ischaemic lesions possibly associated with the vasopressor agents.
The rationale for the selected PET protocol is as follows: PET 1 and PET 2 allow us to assess the influence of anaesthesia on organ blood flow and oxygen consumption. PET 3 and PET 4 examinations allow for the assessment of the effect of vasopressor infusion on organ blood flow and oxygen consumption. The MRI examination is added as a surrogate outcome measure of the vasopressor effects.
PET image acquisition
A Biograph Vision Quadra PET scanner (with a long axial field of view allowing for simultaneous multiorgan assessments of blood flow and oxygen consumption) and PET tracers [15O]H2O and [15O]O2 are used to measure multiorgan blood flow and oxygen consumption parameters.16 23 24
Dosimetry in the study
The total dose of radiation per patient is calculated to be 5.88 mSv.
Outcomes
The primary outcome measure is the between-group difference in the change in CBF (ΔCBF), defined as the difference between post-treatment and pretreatment values (ΔCBF=post-treatment value − pretreatment value).16 17
Secondary outcomes include:
Detection of postoperative cerebral ischaemic lesions: these lesions, potentially linked to vasopressor infusion, will be identified through MRI examinations conducted 24–48 hours postoperatively.
Absolute and relative between-group differences in brain energy consumption parameters such as oxygen extraction fraction and cerebral metabolic rate of oxygen.
Measurements of cerebral tissue oxygen saturation.
Blood flow and oxygen consumption in various organs, including the kidney, liver, myocardium, spinal cord and muscle tissue.
CO assessments and the distribution of CO across different organs.
Evaluation of static cerebral autoregulation.
The secondary outcomes also include the comparisons between diseased and non-diseased brain regions. In this analysis, patients themselves are their own control. We analyse the between-region differences in each patient and pool the results per group. A different vasopressor treatment is treated as a stratification factor.
Data collection and trial monitoring
Study data are collected and managed using REDCap electronic data capture tools hosted at Aarhus University.25 26 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing: (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external sources.
All imaging data are stored on a server with secured and restricted access. Physiological data are initially stored on the respective monitoring devices and subsequently extracted and stored in the study database. The study is conducted according to the standards of Good Clinical Practice and is monitored by the ‘Good Clinical Practice Unit’, Department of Clinical Medicine, Aarhus University Hospital.16 17
PET and MRI analyses
All patients will have a preoperative structural MRI of the brain as part of clinical investigations performed during their preparations for surgery.16 17 T1-weighted MRI of the brain is coregistered with PET images for each patient. Voxel-wise estimation of organ blood flow and oxygen consumption is performed using the arterial blood radioactivity as the input function and a one-tissue compartment model based on previous works.16 27–29 The brain regions of interest are defined as the tumour, peritumoural area and contralateral hemisphere grey matter.16 17 19 Other organs will be delineated using low-dose CT images and analysed using appropriate kinetic models.24
MRI analyses of ischaemic lesions
Diffusion-weighted imaging (DWI) displays regions of reduced water mobility, which is usually ascribed to the regions of cell swelling due to insufficient oxygen availability (ischaemia). To obtain a quantitative measure of the potential harmful effect of the vasopressor, we will detect changes in ischaemic regions by comparing the DWI obtained prior to project enrolment with DWI obtained as a part of the routine postsurgery evaluation. Using machine-learning algorithms, we will automatically segment the prestudy and postsurgery ischaemic regions. Since brain regions potentially alter position during surgery, we will attempt a non-linear coregistration of the two DWI measurements before extracting the prestudy region from the postsurgery region to be able to report the potential volume increase. We will attempt to avoid regions that are the most affected by the surgery, since the surgery itself may also affect the DWI values. This quantitative analysis is supplemented by qualitative inspection.
Sample size and statistical analysis
No previous studies have, to our knowledge, investigated the changes in CBF based on PET measurements after phenylephrine versus norepinephrine treatment in the same patient population. Therefore, we estimated our sample size based on the regional changes in CBF after phenylephrine versus ephedrine treatment in a similar patient cohort as reported by Koch et al.16 Ephedrine and norepinephrine are both combined α-adrenergic and β-adrenergic agonists and have similar pharmacological properties.8 11 16 The study by Koch et al reported a change in CBF of 1.7±3.5 mL/100 g/min after phenylephrine treatment and 5.5±4.0 mL/100 g/min after ephedrine treatment.16 Given a between-group difference in CBF=3.8 mL/100 g/min in favour of the norepinephrine group, a significance level of 0.05 and a power of 80%, the study requires 15 patients in each group to detect a significant difference in CBF changes between the two vasopressors. Considering a possible dropout rate of 6% and to increase the comparability of the two groups, we decided to recruit a total of 32 patients, with 16 patients in each arm.
Statistical analyses
All randomised subjects with a full dataset will be included in the statistical analyses. Standard parametric and non-parametric analyses are used for paired analyses of the physiological parameters.16–19 The percentage changes in different measurements are calculated as the post-treatment value minus pretreatment value divided by the pretreatment value times 100 (ie, the relative change). The effects of vasopressor type, vasopressor dose and CO are estimated in a generalised linear model. Static autoregulation (relationship between MABP and CBF) is assessed using correlation plots and cubic penalised regression splines between norepinephrine and phenylephrine, and between normal and diseased brain regions (in addition to the global CBF). A detailed statistical analysis plan will be prepared prior to the start of the analyses.
Ethics and dissemination
Patients included in this study are required to receive anaesthesia for removal of their brain tumour. Thus, the patients are not exposed to any additional risk due to anaesthesia or placement of intravenous and intra-arterial catheters. The experiment and the removal of the tumour are conducted in the same anaesthesia ‘session’. The experiment will prolong the period for which the patient is under general anaesthesia by approximately 2 hours.16 17 With reference to our previous studies and our experience in general, we do not find that the prolonged period of general anaesthesia nor the PET radiation dose (see Methods section) will pose significant additional risk to the patient.16 17 This is furthermore supported by the obtained approvals from the Danish National Medical Ethics Committee (20 May 2022; 2203674) (Videnskabsetiske Medicinske Komiteer) and the Danish Medicines Agency. Informed consent (online supplemental file 1) will be acquired from the participant prior to study enrolment. Results will be disseminated via peer-reviewed publication and presentation at international conferences. Trial registration number at ClinicalTrials.gov: NCT06083948.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to express our sincere gratitude to anaesthesia nurses Ida Maria Baandrup, Mathilde Juel Kusk and Charlotte Bræmer-Madsen for their invaluable assistance with this study.
References
Footnotes
Contributors NFM has prepared the IMPACT protocol manuscript in close collaboration with MR. The rest of the research group has subsequently reviewed the manuscript. The guarantor is NFM.
Funding The study is funded by the Toyota Foundation (grant/award number: KJ/BG-9983). Mads Rasmussen is funded by the Health Research Foundation of the Central Denmark Region (grant/award number: A4161 MadsRasmussen)
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.