Article Text
Abstract
Objectives To evaluate hospital outcomes and their predictors during the pandemic for patients with and without COVID-19, stratified by the presence of acute kidney injury (AKI).
Design Retrospective observation study using the Hospital Episodes Statistics database for England.
Participants 2 385 337 unique hospital admissions of adult patients from March 2020 to March 2021 in England.
Main outcome measures COVID-19 cases were identified by the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code of U07.1. Patients with suspected COVID-19 (U07.2 code) and patients with end-stage kidney disease on chronic dialysis (N18.6 and Z99.2) were excluded. AKI cases were identified by the ICD10 code. Patients were categorised into four groups based on COVID-19 and AKI diagnoses: Group 1—neither; Group 2—COVID-19 only; Group 3—AKI only; Group 4—both. A multivariable logistic regression model was created with in-hospital mortality as the outcome, including diagnostic groups, demographics, admission methods, comorbidity severity, deprivation index and intensive therapy unit (ITU) admission.
Results Among 2 385 337 admissions involving 663 628 patients, 856 544 had AKI (N17 codes) and 1 528 793 did not. Among patients without AKI, there were 1,008,774 admissions among 133,988 individuals without COVID-19 (Group 1) and 520,019 admissions among 256,037 individuals with COVID-19 (Group 2). Among patients with AKI, there were 630,342 admissions among 218,270 individuals without COVID-19 (Group 3) and 226,202 admissions among 55,333 individuals with COVID-19 (Group 4). Patients in group 4 were older (75.4 ± 13.8 years) and had greater length of stay (17.1 ± 17 days) than all other groups. They also had and had a greater proportion of males, ethnic minorities and comorbidities than other groups. Mortality was highest in Group 4 (28.7%) and lowest in Group 1 (1.1%). The increased risk of death persisted after controlling for multiple baseline factors (OR for death vs Group 1: Group 4—22.28, Group 2—9.67, Group 3—6.44). ITU admission was least required in Group 1 (1.2%) and most in Group 4 (10.9%), with mortality at 4.8% versus 47.8%, respectively.
Conclusions Patients with COVID-19 and AKI have a high risk of mortality and should be recognised early and provided with optimal support. Planning for future pandemics should ensure adequate critical care and acute dialysis capacity.
Trial registration number NCT04579562.
- COVID-19
- Epidemiology
- Health Services
- Acute renal failure
Data availability statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. The authors are unable to fulfil requests for underlying data due to the data sharing agreement with National Health Service (NHS) Digital, which governs the acquisition and analysis of Hospital Episode Statistics (HES) data. Access to HES data can be obtained by directly applying to NHS Digital, subject to their conditions of use and further usage policies.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
National data on hospitalised COVID-19 patients addresses a key gap by comparing outcomes across multiple cohorts, including a control group, to better understand varying risks.
Reliance on diagnosis via administrative codes may lack granularity and accuracy.
Administrative data reflects real-world clinical practices.
The observational design limits causal interpretations.
Introduction
While the primary focus of COVID-19 was initially on its respiratory effects, it soon became evident that severely and critically ill patients were at heightened risk of developing acute kidney injury (AKI), a condition associated with a higher mortality rate and poorer prognosis.1–3 In response to this concern, researchers worldwide have published numerous reports examining the incidence, risk factors and clinical outcomes. However, the occurrence of AKI among hospitalised COVID-19 patients has shown variability with studies from the UK reporting AKI rates ranging from 26.2% to 31.5%, whereas reports from the USA have indicated even higher rates, ranging from 37% to 57%.1 2 4 5 The perception that AKI in COVID-19 may be more severe than AKI from other causes has prompted studies to compare AKI associated with COVID-19 with AKI in various other viral infections with conflicting results.6 7 Some have found that the incidence of AKI was comparable to other viral infections, while others have reported a higher incidence of AKI and more adverse outcomes in patients hospitalised with COVID-19. However, research on AKI related to COVID-19 has been constrained by relatively small sample sizes, limited geographical representation and variable duration of observation.8 Few studies have directly compared AKI cases in non-COVID-19 illnesses with those in COVID-19 patients during the same period.1 5 Furthermore, there is a lack of comprehensive national-level studies to address geographical and temporal disparities. Additionally, while both COVID-19 and AKI have been associated with increased mortality, the interaction between these conditions has not been thoroughly investigated, particularly with appropriate control groups. This comprehensive national study endeavours to fill this knowledge gap by comparing outcomes in people admitted to hospitals during the pandemic with and without COVID-19, stratified by the presence or absence of AKI and controlled for demographic and health characteristics. Importantly, a control group of patients hospitalised during the same period without AKI or COVID-19 has been incorporated into the analysis. A secondary objective was to assess predictors of in-hospital mortality.
Methods
Ethical and regulatory approval
The study was reviewed and approved by University Hospitals of Derby and Burton National Health Service (NHS) Foundation Trust’s research and development department. Informed consent was waived due to the nature of the study and the pandemic situation. The study was registered on the National Library of Medicine website (www.clinicaltrials.gov) with registration number NCT04579562, and the protocol is available in online supplemental text 1. The study was conducted following the principles of the Declaration of Helsinki and followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines (online supplemental table S5).
Supplemental material
Study design and procedure
We obtained anonymised data from the Hospital Episode Statistics (HES) database, which includes detailed records of all patients admitted to any hospital in England commissioned by the NHS. Patients from outside England were excluded. We included all adult patients aged 18 years and above, who were admitted to NHS hospitals in England between 1 March 2020 and 31 March 2021, and had a diagnostic code for COVID-19 included in any of their 20 diagnosis codes. This study period encompassed all admissions during the period when the SARS-CoV-2 virus was classified as ‘Original’ and later, when the ‘Alpha’ strain of SARS-CoV-2 became predominant. Each patient’s data were recorded at the Finished Consultant Episode (FCE) level, which represents the care delivered by a single consultant. In the context of hospital records, a ‘spell’ refers to a whole hospital stay, including all related treatments and services. The records of these stays are kept as FCEs, and each individual hospitalisation or ‘spell’ can have multiple FCEs associated with it. To obtain details of the intensive therapy unit (ITU) stay, we linked the admitted patient care dataset with the critical care dataset, including the total number of ITU stays and total organ support. We also linked the dataset to the Office of National Statistics dataset to obtain the date of death for each patient.
Definitions
We used the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code of U07.1 to identify episodes of confirmed COVID-19 between 1 March 2020 and 31 March 2021, by extracting only one FCE from each spell or admission containing the U071 code. We excluded episodes with the U07.2 code (suspected COVID-19) and patients with end-stage kidney disease on chronic dialysis (N18.6 and Z99.2). We identified AKI by ICD10 code in any of the 20 diagnoses codes and extracted up to 20 diagnoses codes and 24 OPCS-4 codes, including codes for dialysis (online supplemental table S1) in the same spell, to ensure temporal relation between COVID-19 and AKI. We grouped ethnicity into six categories and English IMD quintiles were summarised as a categorical factor. Comorbidities studied included myocardial infarction, congestive cardiac failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic lung disease, connective tissue disorder, diabetes with complications, paraplegia, chronic kidney disease, chronic liver disease and cancer. Charlson’s Comorbidity Index (CCI) was computed using ICD-10 hospitalisation codes (online supplemental table S2) and categorised based on the total score into four categories: no comorbidity with CCI score of 0; mild, with CCI scores of 1–2; moderate, with CCI scores of 3–4; and ≥5 was categorised in the severe category.9–11 The study included admission methods as elective, emergency, maternity and child, transfers and unknown.
Outcomes
The primary aim of the study was to analyse and compare outcomes in two groups of patients hospitalised with COVID-19: those with AKI and those without AKI. A secondary aim was to compare outcomes in patients hospitalised with COVID-19 and AKI to those with AKI from other causes. In addition, a control group of patients who were hospitalised without AKI or COVID-19 was included for reference. In addition, we aimed to explore how demographic and health characteristics might impact in-hospital mortality rates to inform future strategies to improve outcomes.
Statistical analysis
We used IBM SPSS Statistics for Windows, V.28.0 to analyse our data. Each admission had a binary outcome, so we analysed the data for each admission of AKI separately. We included patients who had multiple admissions of AKI in separate admission periods to prevent survival bias by analysing the first admission or mortality bias by analysing the last admission. Analysis of variance was used to compare continuous variables, reported as mean with SD, while χ2 or Fisher’s exact test was used to compare categorical variables, reported as proportions and percentages. Since there were few missing data, we did not perform multiple imputations. We categorised patients into four groups according to whether or not they had a diagnosis of COVID-19 and a diagnosis of AKI: Group 1—no COVID-19 or AKI; Group 2—COVID-19 without AKI; Group 3—AKI without COVID-19; Group 4—COVID-19 with AKI. We created a multivariable logistic regression model with in-hospital mortality as the outcome and included diagnostic groups, demographic variables, admission methods, comorbidity severity, index of multiple deprivations and ITU admission. We performed sensitivity analyses to confirm our findings using individual comorbidities instead of CCI from the model (online supplemental table S4). ORs and 95% CIs were used to present the results, and p<0.05 was considered statistically significant.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
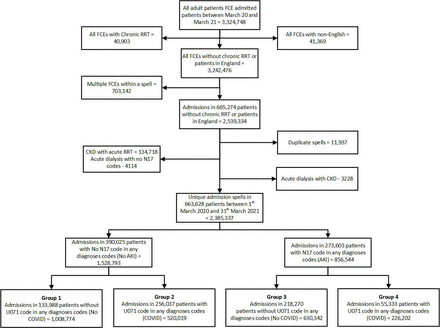
We extracted 3 324 748 FCEs of all adult patients admitted between March 2020 and March 2021 from HES—England’s national database of all hospital admissions (figure 1). We excluded multiple FCEs within the same spell and duplicate FCEs. Out of 2 385 337 unique admission spells in 663 628 patients, there were 856 544 admission spells (35.91%) in 273 603 patients with AKI as identified by N17 codes in any of the 20 diagnostic codes, while 1528 793 had no AKI. Among 746 221 admissions with COVID-19, 226 202 (30.31%) admissions had AKI. There were 1 008 774 admissions in 133 988 patients who did not have AKI or COVID-19 (Group 1) and 520 019 admissions in 256 037 patients who had COVID-19 without AKI (Group 2). Among admissions with AKI, there were 630 342 admissions in 218 270 patients who did not have COVID-19 (Group 3) and 226 202 admissions in 55 333 patients with COVID-19 and AKI (Group 4).
Study flowchart. AKI, acute kidney injury; CKD, chronic kidney disease; FCE, finished consultant episode; RRT, renal replacement therapy.
Patient data according to diagnostic groups are shown in table 1. The incidence of AKI was 35.91% in the whole cohort and 30.31% in patients with COVID-19. Patients who had AKI were older than patients in non-AKI groups. Patients in Group 4 were significantly older (75.4±13.8 years), predominantly of male gender (58.9%), had higher emergency admission rates (96.4%) and a higher proportion of black ethnicity (4.7%) compared with all other groups. There was a higher proportion of patients with Asian ethnicity in both the COVID-19 groups (Group 2—8.3% and Group 4—7.0%), as compared with the non-COVID-19 groups (Groups 1 and 3). There was a greater proportion of patients in the decile with the highest deprivation compared with the decile with the lowest deprivation in all four groups. There were small but statistically significant differences in social deprivation between the groups. While all four groups had a greater proportion of patients in the category of mild comorbidities, patients in Group 4 had a significantly higher proportion of moderate comorbidities as compared with all other groups. Group 4 had a significantly higher proportion of patients with cerebrovascular disease (11.7%), dementia (17.8%), kidney disease (37%) and diabetes (35.9%) as compared with all other groups (online supplemental table 3).
Demographic characteristics and outcomes of patients hospitalised with and without acute kidney injury (AKI) and COVID-19 in England
Patients in Group 4 had the longest duration of hospitalisation, with an average length of stay (LOS) of 17±17 days, while the control group (Group 1) had the shortest LOS at 4.3±10.1 days, p<0.001. Additionally, a larger proportion of Group 4 patients (3.4%) had kidney replacement therapy (KRT) compared with Group 3 (1.4%), p<0.001. Unadjusted mortality was highest in patients in Group 4 (28.7%), followed by Group 2 (10.9%), Group 3 (10%) and lowest in Group 1 (1.1%), p<0.001 (table 1).
ITU admissions
In Group 4, 10.9% of patients were admitted to ITU (table 2), followed by Group 3 (5.3%), Group 2 (5.1%) and Group 1 (1.2%), p<0.001. The number of organs supported differed significantly with patients in Group 4 (2.8±1.6) and Group 3 (2.2±1.5) needing more organ support, p<0.001. Patients in Group 4 needed significantly more days of advanced respiratory support (11.2±14.0 days, p<0.001), more days in ITU (11.5±14.3 days, p<0.001) and a higher number of days of kidney support as compared with Group 3 (3.3±7.7 days vs 1.4±4.3 days, p<0.001). ITU mortality differed significantly between all four groups, with the highest mortality in Group 4 at 47.8% followed by Group 2 at 22.3% and the lowest mortality in Group 1 at 4.8%, p<0.001.
Intensive therapy unit (ITU) characteristics of patients hospitalised with and without acute kidney injury (AKI) and/or COVID-19 in England
Associations with in-hospital mortality
A multivariable logistic regression was performed to ascertain the effects of age, gender, ethnicity, admission method, CCI, ITU admission, IMD and the diagnostic groups of AKI and COVID-19 on the likelihood of case fatality. In comparison with Group 1, COVID-19 complicated by AKI (Group 4) demonstrated the highest likelihood of death after correction for multiple potential confounders (OR 22.28, 95% CI 21.79, 22.78) followed by those with COVID-19 only (Group 2: OR 9.67, 95% CI 9.46, 9.88) (figure 2 panel A) and those with AKI only (Group 3: OR 6.44, 95% CI 6.30, 6.58). Age (OR 1.04, 95% CI 1.04, 1.04), unknown ethnicity (OR 1.08, 95% CI 1.06, 1.11), transfers from other hospitals (OR 3.36, 95% CI 3.20, 3.53) and ITU admission (OR 6.36, 95% CI 6.25, 6.48) also had higher odds of death (figure 2 panel B). Additionally, a greater comorbidity burden was linked to an increased likelihood of mortality. When compared with no comorbidities, the odds of death increased progressively for mild comorbidities (OR 1.61, 95% CI 1.59, 1.64), moderate comorbidities (OR 2.07, 95% CI 2.03, 2.11) and severe comorbidities (OR 3.00, 95% CI 2.94, 3.07). Sensitivity analysis using individual comorbidities instead of CCI and SARS-CoV-2 variant showed similar findings (online supplemental table S4). In both analyses, increasing CCI was an independent risk factor for higher mortality.
Multivariate analysis of predictors of mortality in patients hospitalised with COVID-19. AKI, acute kidney injury; CCI, Charlson’s Comorbidity Index; ITU, intensive therapy unit.
The analysis revealed that as the levels of deprivation increased, the likelihood of mortality also increased when compared with the least deprived group (figure 2 panel C). However, this relationship did not demonstrate a consistent progression.
Discussion
In this large national observational study, we observed that COVID-19 complicated by AKI was associated with substantially higher in-hospital mortality than either COVID-19 without AKI or AKI due to other causes, and that this association persisted after controlling for multiple potential confounders. We also noted that COVID-19 patients with AKI were more frequently in need of mechanical ventilation and advanced cardiac support and had a higher ITU mortality rate compared with the other three groups. Our analysis has identified risk factors for the development of AKI with COVID-19 and increased mortality, which will aid risk stratification and prioritisation of clinical care. Moreover, these data will be valuable in planning for an adequate response to future pandemics.
Several mechanisms have been proposed to account for the association between COVID-19 and AKI including factors like reduced oxygen levels, low blood pressure, inflammation, blood clot formation, an excessive release of cytokines, severe sepsis and endothelial dysfunction.12–14 Additionally, a direct viral infection of kidney cells has been suggested as a cause of AKI in COVID-19, supported by the detection of viral RNA, viral protein and live virus in kidney tissue in some studies.15–18 A recent autopsy study found a connection between the presence of viral RNA in the kidneys and clinical outcomes, including AKI, although how much this contributes to AKI in hospitalised COVID-19 patients is still uncertain.19
We observed a slightly lower prevalence of AKI (30.31%) in patients hospitalised with COVID-19 versus those without COVID-19 (35.91%). This is in contrast with our own single-centre study conducted during the early stages of the pandemic, which identified a higher incidence of AKI among individuals hospitalised with COVID-19 (26.2%) versus those without COVID-19 (12.4%).1 Similarly, in the USA, the incidence of AKI was greater in the group of individuals with COVID-19 when compared with those without COVID-19 (57% vs 37%, respectively).5 The reasons for this disparity are not clear but may include a higher threshold for admitting patients without COVID-19 during the pandemic and regional variation.
Our observations align with prior research, showing that hospitalised individuals with COVID-19 and AKI tend to be older, are predominantly male and exhibit moderate to high levels of comorbidities.20–22 We also noted that patients with COVID-19, whether with or without AKI, had a higher percentage of individuals from Asian and black ethnic backgrounds compared with those without COVID-19. Additionally, a greater proportion of COVID-19 patients, regardless of AKI status, were from socioeconomically deprived areas. These risk factors may be clinically helpful to identify patients with COVID-19 who are at greater risk of developing AKI and may benefit from closer monitoring.
We found that the unadjusted mortality rate for patients with both COVID-19 and AKI was more than double that for COVID-19 without AKI or AKI without COVID-19. Importantly, the increased risk of death in patients hospitalised with COVID-19 and AKI persisted after adjustment for multiple other risk factors in a multivariable analysis. In Northern Italy, COVID-19-related AKI was linked to 59.7% of deaths, while in Spain, mortality was 38.5%, compared with 13.4% in the overall population during wave 1.23 24 This implies that in the context of COVID-19, AKI is not simply a consequence of disease severity but actively contributes to substantially increasing mortality. Our observations are consistent with previous studies that have reported a higher fatality rate among COVID-19 patients with AKI (33.3–86.4%), contrasting with COVID-19 without AKI (5.6–9.3%).2 5 25–27 Moreover, the inclusion of appropriate control groups enables us to provide robust evidence that AKI associated with COVID-19 is associated with a much higher risk of death than AKI due to other causes. In practice, this means that clinicians should recognise patients with COVID-19 and AKI to be at particularly high risk of death and ensure that they receive close monitoring and early referral for ITU support and/or RRT.
This study has several strengths compared with previously published reports. Prior observational studies have individually explored the risk factors and outcomes of AKI among COVID-19 patients who were hospitalised.1 20–22 28 However, there have been no comprehensive large-scale systematic studies that compare the outcomes of COVID-19 patients with AKI to those without AKI, as well as to individuals with AKI in the absence of COVID-19, and patients admitted without COVID-19 and AKI during the same observation period. Nevertheless, several limitations should be considered. First, we relied on administrative codes for diagnosis, which have limitations when it comes to accurately capture and characterising AKI. However, we believe that this is outweighed by the advantage of administrative codes encompassing a vast amount of data from multiple healthcare institutions, providing researchers with access to a large and diverse patient population. Second, we specifically considered patients with laboratory-confirmed diagnoses of COVID-19, but it is worth noting that there may have been individuals in the early stages of the pandemic who were infected with SARS-CoV-2 but were not included in the study due to limited testing availability. Third, we did not have access to clinical data regarding the physiological status of patients which may have impacted the outcomes. Finally, we did not have vaccination data and are unable to determine any impact of vaccination on the incidence of AKI.
Conclusions
Patients with COVID-19 should be proactively monitored for the development of AKI and managed to reduce the incidence of AKI, particularly if they are in identified high-risk groups. Those who develop AKI should be recognised as having a very high risk of mortality and provided with optimal treatment and supportive care from early in their course. Moreover, our observations reinforce the message that prevention through vaccination remains important for at-risk groups. In planning for future pandemics, healthcare systems should consider the broader impact of an emerging infection. This should include the provision of sufficient critical care and acute dialysis capacity to adequately support the anticipated number of cases of acute infection and AKI.
Data availability statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. The authors are unable to fulfil requests for underlying data due to the data sharing agreement with National Health Service (NHS) Digital, which governs the acquisition and analysis of Hospital Episode Statistics (HES) data. Access to HES data can be obtained by directly applying to NHS Digital, subject to their conditions of use and further usage policies.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants, but Health Research Authority and Wales Research Ethics Committee exempted this study (HRA Reference: 20/HRA/4002).
Acknowledgments
We acknowledge NHS Digital for permission to use their data in this report. We also thank Lindsay Snell for the literature search
References
Footnotes
NVK, RF and MT contributed equally.
Contributors This study was designed and organised by NVK, RF and MT. Data cleaning and analysis were done by NVK. Writing of the first draft was by NVK and MT. NVK is the guarantor. All authors critically reviewed the manuscript and agreed to submission of the final draft.
Funding There was no funding for this study
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/disclosure-of-interest (available on request from the corresponding author) and declare the following: No funding for the study; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work. The manuscript’s guarantor (NVK) affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.