Article Text
Abstract
Introduction The standard first-line treatment for driver-gene negative advanced non-small cell lung cancer (NSCLC) is chemotherapy combined with immunotherapy. However, owing to the immune microenvironment imbalance and immune status impairment caused by repeated chemotherapy, as well as the primary or secondary resistance to immune checkpoint inhibitors, the efficacy of immunotherapy combined with chemotherapy remains unsatisfactory. Recent studies have shown that faecal microbiota transplantation (FMT) can modulate the intestinal microflora, influence the tumour immune microenvironment and even enhance the efficacy of immunotherapy. Hence, we conduct such a prospective, exploratory study to evaluate the efficacy and safety of integrating FMT with standard first-line treatment in patients with driver-gene negative advanced NSCLC.
Methods and analysis FMT-JSNO-02 (NCT06403111) is a prospective, multicentre, single-arm exploratory study. It is planned to include 62 cases of previously untreated driver-gene negative, Eastern Cooperative Oncology Group Performance Status 0–1, programmed death ligand 1<50% advanced NSCLC patients, who will be given FMT by orally ingested stool capsules on the basis of standard first-line treatment of chemotherapy combined with immunotherapy. The primary endpoint of this study is the 12-month progression-free survival rate.
Ethics and dissemination The study was approved by the ethics committee of the Second People’s Hospital of Changzhou (number [2024] YLJSA005) and is being conducted in accordance with the principles of the Declaration of Helsinki. The results of this study will be disseminated through publication in a peer-reviewed journal and presentation at scientific conferences.
Trial registration number NCT06403111. Date of registration: 7 May 2024, the first version protocol.
- CHEMOTHERAPY
- Clinical Relevance
- Clinical Trial
- ONCOLOGY
- Respiratory tract tumours
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is a multicentre prospective study.
Patients receive faecal microbiota transplantation in a more convenient and acceptable capsule form.
The sample size in this study is relatively small.
This is a single-arm study rather than a randomised controlled study.
Introduction
Current situation and dilemmas of immunotherapy for non-small cell lung cancer (NSCLC)
Lung cancer is one of the most prevalent malignancies globally and the major driver of cancer mortalities, responsible for 18.7% of all cancer fatalities.1 As the primary pathological subtype of lung cancer, most NSCLC patients present with local spread or distant metastasis, often missing the chance for surgery and facing poor prognosis. Recently, immune checkpoint inhibitors (ICIs), especially those targeting the programmed death receptor 1 (PD-1) and its ligand (PD-L1), have markedly improved treatment outcomes and become an effective treatment strategy for NSCLC.
Currently, the combination of chemotherapy and immunotherapy is the established standard first-line treatment for patients with advanced NSCLC owing to extensive clinical studies conducted on pembrolizumab and nivolumab. Tislelizumab developed in China has shown remarkable efficacy. In patients with lung adenocarcinoma, the addition of tislelizumab to chemotherapy resulted in a notably lengthened median progression-free survival (mPFS) compared with chemotherapy alone (9.7 vs 7.6 month), with a 12-month PFS rate of 31.3%.2 Similarly, in patients with lung squamous cell carcinoma, the mPFS for the tislelizumab plus chemotherapy group was also higher than that of chemotherapy alone (9.6 months vs 5.5 months).3
Despite the notable improvement in survival outcomes for advanced NSCLC patients achieved by ICIs, primary or acquired immune resistance inevitably arises in clinical practice. Existing research suggests that primary immune resistance is related to the dysfunction in tumour antigen handling and presentation, insufficient T-cell infiltration in the tumour microenvironment (TME) and overexpression of suppressive immune cells.4 Acquired immune resistance is related to adaptive changes that occur in tumour cells and the TME during exposure to immunotherapy.5
Nowadays, a growing body of research suggests that gut flora may be able to reverse immune resistance. For instance, gut microbiota can regulate the infiltration of immune cells in the TME by modulating innate and adaptive immunity. They can also reprogramme the TME through their reactive metabolites or secretions, affecting the efficacy of ICIs and even reversing immune resistance.6 Researchers have conducted preliminary explorations into how gut microbiota affects the efficacy of immunotherapy. Zitvoge discovered that the antitumour effects of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade were reliant on certain Bacteroides sp. The antitumour effect of CTLA-4 blockade was absent in antibiotic-treated or germ-free mice. The issue was resolved by giving Bacteroides fragilis via gavage, immunising with its polysaccharides or transferring B. fragilis-specific T cells.7 At the same time, Sivan et al found that mice orally administered Bifidobacterium could achieve the same antitumour effect as PD-L1 monoclonal antibodies, and the combination almost completely inhibited tumour growth.8 Thus, regulating gut microbiota holds promise for enhancing therapeutic efficacy in patients undergoing immunotherapy.
Current status of faecal microbiota transplantation (FMT)
The gut microbiota is susceptible to various factors, including the use of broad-spectrum antibiotics, repeated chemotherapy and other treatments. Common methods to regulate the intestinal microbiota include dietary intervention, probiotics, prebiotics and FMT. Among these approaches, FMT stands as the most established technique.9 FMT is the transplantation of functional flora from the stool of a healthy person into the intestine of a patient in a certain way. This method mainly includes two ways: injecting microflora fluids and swallowing capsules. Among them, FMT by orally ingested stool capsules (capsulised FMT) has the characteristics of a wide range of application, high acceptance degree, relative economy and convenience.10
The relationship between intestinal flora and immunotherapy efficacy has been preliminarily confirmed in some clinical studies of malignant tumours. For example, in a study investigating 15 melanoma patients who were unresponsive to anti-PD-1 therapy, rechallenge with FMT and pembrolizumab showed objective responses in 3 out of 15 patients, and these 3 patients maintained stable disease (SD) for over 12 months.11 In a clinical study (NCT04264975), investigators conducted FMT with ICIs in the treatment of 13 patients with advanced solid tumours resistant to ICIs. The findings revealed that FMT led to lasting microbiota alterations and clinical improvements in 6 patients, resulting in an objective response rate (ORR) of 7.7% and a disease control rate of 46.2%.12 As a result, FMT holds great potential to reverse immune resistance and enhance the efficacy of immunotherapy.
Hence, we conduct such a prospective, exploratory study to evaluate the efficacy and safety of integrating FMT with standard first-line treatment in patients with driver-gene negative advanced NSCLC. We expect that the addition of FMT to the standard first-line treatment in NSCLC will be safe and will have an enhancing effect.
Methods
Study design
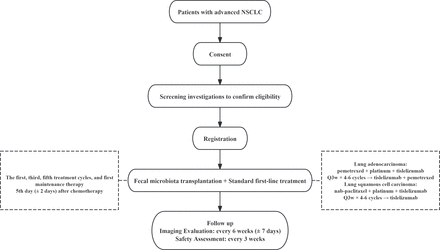
This study is designed to evaluate the efficacy and safety of FMT combined with standard first-line treatment of patients with advanced NSCLC who are driver-gene negative. The trial protocol was written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials guidance. The study will mainly be conducted at the Second People’s Hospital of Changzhou, the Third Affiliated Hospital of Nanjing Medical University. Recruitment information can be obtained through outpatient consultations, posters displayed in the wards and other channels. A total of 62 patients with driver-gene negative, Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–1 and PD-L1<50% who have not received prior treatment are expected to be included in this study without grouping. Patients will receive platinum-based doublet chemotherapy combined with tislelizumab as standard first-line treatment for 4–6 cycles and then enter maintenance therapy. FMT will be given to patients during the first, third and fifth treatment cycles, as well as during the first maintenance therapy. All the enrolled patients or their designated agents will sign the informed consent form within 3 days before starting treatment (online supplemental file 1). See figure 1 for details.
Supplemental material
The participant pathway of the clinical trial. NSCLC, non-small cell lung cancer.
Patient population
Inclusion criteria
The subjects voluntarily joined the study and were able to sign the informed consent with good compliance.
Age 18–80 years old (when signing the informed consent form).
Patients with histologically or cytologically proven locally advanced (IIIB/IIIC), metastatic or recurrent (stage IV) NSCLC who are inoperable and unable to receive radical concurrent chemoradiotherapy, according to the International Association for the Study of Lung Cancer and the American Joint Committee on Cancer Classification, 8th Edition TNM Classification of Lung cancer.
Have not received systemic intravenous antitumour therapy before, and the driver gene is negative.
PD-L1 expression<50%.
According to the solid tumour efficacy evaluation criteria (RECIST V.1.1), there is at least one radiographically measurable lesion. That is, in CT or MRI detection, the longest diameter of a single lesion was ≥10 mm, or the pathological enlargement of a single lymph node was ≥15 mm.
The physical status score of the Eastern Tumor Collaboration Group (ECOG) was 0–1.
Expected survival >3 months.
Have adequate organ and bone marrow function, laboratory tests within 7 days prior to enrolment meet the following requirements (no blood components, cell growth factors, albumin or other corrective drugs are allowed within 14 days prior to obtaining laboratory examination), as follows: (1) Blood routine: absolute neutrophil count≥1.5×109/L, platelet≥75×109/L, haemoglobin≥90 g/L (no blood transfusion or erythropoietin dependence within 14 days). (2) Liver function: serum total bilirubin≤2 times the upper limit of normal (ULN). Alanine aminotransferase and/or aspartate aminotransferase≤5× ULN, serum albumin≥28 g/L, alkaline phosphatase≤5× ULN. (3) Renal function: serum creatinine (Cr)≤1.5× ULN, or Cr clearance≥50 mL/min (using the standard Cockcroft-Gault formula): Urine routine results showed urinary protein<2+. For patients with urine protein≥2+ at baseline, 24-hour urine collection and 24-hour urine protein quantification<1 g should be performed. (4) Coagulation function: international standardised ratio (INR) or prothrombin time≤1.5 times ULN. If the subject is receiving anticoagulant therapy, as long as the INR is within the intended range of anticoagulant drug use.
For female subjects of reproductive age, a urine or serum pregnancy test should be performed and the result should be negative 3 days prior to receiving the initial study drug administration.
Subjects and their sexual partners are required to use a medically approved contraceptive method (such as an intrauterine device, contraceptive pill or condom) during the study treatment period and for 6 months after the end of the study treatment period.
Exclusion criteria
Currently participating in an interventional clinical study or receiving another investigational drug or investigational device within 4 weeks prior to initial dosing.
Received proprietary Chinese medicines with antitumour indications or immunomodulatory drugs (thymosin, interferon, interleukin, etc) within 2 weeks before the first administration or received major surgical treatment within 3 weeks before the first administration.
Class III–IV congestive heart failure (New York Heart Association classification), poorly controlled and clinically significant arrhythmias.
Any arterial thrombosis, embolism or ischaemia, such as myocardial infarction, unstable angina pectoris, cerebrovascular accident or transient ischaemic attack, occurred within 6 months before treatment.
Known allergic reaction to the drug in this study.
Patients who require long-term oral, intravenous or intramuscular administration of systemic corticosteroids.
Symptomatic central nervous metastases. Patients with asymptomatic brain metastases (BMs) or BMs whose symptoms are stable after treatment are eligible to participate in this study if they meet all of the following criteria: measurable lesions outside the central nervous system. No midbrain, pontine, cerebellum, meninges, medulla oblongata or spinal cord metastasis. Maintain clinical stability for at least 2 weeks. Stop hormone therapy 3 days before the first dose of the study drug.
There is an active infection requiring treatment or systemic anti-infective drugs have been used in the week prior to the first dosing.
Has not fully recovered from toxicity and/or complications caused by any intervention before starting treatment (ie, ≤ grade 1 or baseline, excluding weakness or hair loss).
Known history of HIV infection (ie, HIV 1/2 antibody positive).
Untreated active hepatitis B (defined as HBsAg positive and HBV-DNA copy number detected greater than the ULN value in the laboratory of the study centre).
Active HCV-infected subjects (HCV antibody positive and HCV-RNA levels above the lower limit of detection).
Received live vaccine within 30 days prior to the first dose (cycle 1, day 1). Note: injectable inactivated virus vaccine against seasonal influenza is permitted for 30 days prior to initial administration. However, live attenuated influenza vaccines administered intranasally are not permitted.
Pregnant or lactating women.
Medical history or evidence of disease that may interfere with test results, prevent participants from fully participating in the study, abnormal treatment or laboratory test values or other conditions that the investigator considers unsuitable for enrolment. The investigator considers other potential risks unsuitable for participation in the study.
Treatment regimen
Dose selection: tislelizumab: 200 mg, pemetrexed: 500 mg/m2, albumin-bound paclitaxel: 260 mg/m2, platinum drugs (cisplatin: 60–80 mg/m2; carboplatin: area under curve (AUC) =5; nedaplatin: 80 mg/m2). Capsulised FMT: 30 capsules each time. Capsules will be stored in the refrigerator at −80°C. When taking capsules, take them out of the refrigerator, heat them in a water bath (single-hole water bath, JF-01 defroster) at 37°C for 10 min and swallow them with warm water.
Participants will receive FMT combined with tislelizumab+pemetrexed+platinum-based treatment (lung adenocarcinoma)/albumin-bound paclitaxel+platinum-based treatment (lung squamous cell carcinoma) for 4–6 cycles. If there is no progression of the disease after 4–6 cycles of the first-line treatment, then patients will enter the maintenance treatment stage. Patients will receive tislelizumab maintenance treatment (lung squamous cell carcinoma) or tislelizumab+pemetrexed maintenance treatment (lung adenocarcinoma). Treatment continues until disease progression, subject withdraws informed consent, loss of follow-up or death.
Capsulised FMT should be conducted on the 5th day (±2 days) after the start of the first, third and fifth treatment cycles of chemotherapy, as well as on the 5th day (±2 days) after the first maintenance chemotherapy (lung adenocarcinoma) and before the first maintenance immunotherapy (lung squamous cell carcinoma). All patients will be prohibited from receiving any other treatments with antitumour activity or potential antitumour activity during the study. This will be monitored by the investigators, the patients and their families. See figure 2 for details.
Timeline for FMT. ECOG, Eastern Cooperative Oncology Group; FMT, faecal microbiota transplantation; PD-L1, programmed death ligand 1; NSCLC, non-small cell lung cancer.
Study endpoints
The primary endpoint of the study is the 12-month PFS rate. Secondary endpoints include ORR, mPFS, overall survival (OS), duration of response, safety, microbiome diversity and quality of life (QoL). Exploratory endpoints including efficacy predictive biomarkers, including fungi, bacteria, metabolomics and proteomics.
Sample size estimation
The parameters are set as follows: the two-sided significance level alpha is set at 0.05. Based on an estimated dropout rate of 0% and a reference 12-month PFS rate of 35% published in the RATIONALE-304 and RATIONALE-307 clinical studies, it is inferred that the 12-month PFS rate in this trial can reach 55%.2 3 Therefore, a total sample size of 62 participants is determined for this study to ensure statistical power greater than 80%.
Efficacy and safety assessments
Imaging evaluation
Screening period evaluations must be completed within 28 days before the initial administration of the study drug. Prior to treatment, researchers at the study centre will confirm that subjects have measurable lesions that meet the RECIST V.1.1 criteria. The methods used to assess tumour burden at baseline must be consistent with those used for each subsequent follow-up assessment (CT/MRI). Additional imaging assessments of other suspected involved areas (eg, brain) may be conducted based on the subjects’ clinical symptoms and signs. After enrolment, tumour status will be evaluated using imaging methods every 6 weeks (±7 days), every 12 weeks (±7 days) after 48 weeks until disease progression (RECIST V.1.1) or death (during the course of patient treatment). For patients who complete or discontinue treatment for non-progression reasons, a single imaging assessment will be conducted at treatment end/discontinuation.
Safety evaluation
Researchers will conduct safety assessments on patients every 3 weeks using NCI-CTCAE V.5.0. After treatment ends, participants will be followed up for 30 days to detect adverse events (AEs). If the patient has not received new antitumour treatment within 90 days after the last dose, severe AEs (SAEs) occurring within 90 days after the last dose will be collected. If the subject has received new antitumour treatment, SAE prior to the new treatment will be collected, with precedence given to those that have already occurred. Investigators should grade and record AEs for each subject according to the NCI-CTCAE V.5.0 criteria during the study and follow-up period. The characteristics of AEs will be assessed and recorded based on severity, causality, toxicity grading, management measures and outcomes.
Faecal and peripheral blood collection time
With the permission of the ethics committee, patients should provide 10 mL whole blood samples and faecal samples at baseline, after two cycles of treatment, before maintenance treatment and after two cycles of maintenance treatment for the detection of efficacy prediction markers (each cycle is 21 days).
Data management
Data management methods
The study will use a data collection system (91trial) for data management. All subjects will be assigned a unique ID. Researchers will input basic information, drug use, laboratory examination and other raw data information of subjects into 91trial. The system is subject to superior monitoring and cannot be arbitrarily modified. The data manager will write a data audit report based on the trial protocol and audit criteria in the database. The results of the patients’ report on tests, examinations, etc can be obtained from the electronic case report. QoL will be evaluated through the EORTC-QLQ-C30 Questionnaire, which will be available in both paper and electronic formats.
Throughout the data collection process, researchers will implement preventive measures to ensure the confidentiality of the documents and safeguard against the identification of participants. All data will be monitored by the data monitoring committee.
Reporting and collection of AEs
When a clinical AE occurs, it will be detailed on the case report form with the time of occurrence, clinical manifestations, course of treatment, duration, outcome and the relationship to this study. For those with laboratory abnormalities, follow-up will continue until the test results return to normal, or to the level before medical intervention or until it is determined that the event is unrelated to the medical intervention. In the event of an SAE, the SAE form should be filled out and reported to the department and relevant functional departments within 24 hours and recorded in the hospital quality control system, with documentation in the medical record.
Statistical analysis
This study will use SPSS V.27.0 statistical software for data analysis. By recording patients’ survival times, Kaplan-Meier survival curves for PFS%, PFS and OS will be plotted to visually display the trend of survival rates over time. The occurrence of AE and SAE will be recorded, and the frequency and percentage of different levels of AEs will be used to describe their occurrence. The Log-rank test will be used to compare whether there are statistically significant differences between the historical control group and the experimental group.
Subgroups will be divided based on patients’ demographic characteristics (age, sex), tobacco use, nutritional status, ECOG PS, pathological features (tumour type, solid tumour stage, pathological subtype), metastatic sites (such as brain metastasis, liver metastasis), PD-L1 expression and other relevant factors. A multivariate analysis of PFS and OS will be performed using the Cox proportional hazards model. A p value of <0.05 will be considered statistically significant.
Trial status
The study was registered at ClinicalTrials.gov (NCT06403111) on 7 May 2024, and patient recruitment is ongoing. The first participant was enrolled on 25 June 2024, with the study anticipated to conclude by 1 June 2026.
Patient and public involvement
None.
Ethics and dissemination
The study was approved by the ethics committee of the Second People’s Hospital of Changzhou (number [2024] YLJSA005) and is being conducted in accordance with the principles of the Declaration of Helsinki. The study was registered in ClinicalTrials.gov (NCT06403111). Significant protocol changes will be notified to involved parties and updated on ClinicalTrials.gov. Before participating in the study, patients or their legally authorised representatives will provide written informed consent. Throughout the study, they will be informed of any new details that could influence their decision to remain in the trial. They can withdraw at any time without facing any penalties or losing any benefits to which they are entitled. If participants suffer harm owing to research interventions or research-related procedures during the trial, appropriate compensation will be provided in accordance with relevant laws, regulations and the guidance of the ethics committee. The results of this study will be disseminated through publication in a peer-reviewed journal and presentation at scientific conferences.
Discussion
The application of ICIs has brought significant benefits to patients with driver-gene negative NSCLC, but statistics show that the 5-year survival rate for advanced NSCLC patients receiving immunotherapy is only about 20%.13 As research into the mechanisms of immune resistance advances, researchers have discovered that the gut microbiota plays a pivotal role in modulating the immune system.
Preclinical studies have found that specific gut microbiota or FMT using faeces from patients who respond to ICIs can modulate the immune system, enhance immune cell infiltration in tumours, induce tumour regression and improve the antitumour efficacy of ICIs.8 14–16 Existing clinical trials have shown that exogenous microflora supplementation by FMT can change the composition of intestinal microflora and the proportion of dominant microflora, reprogram the TME and potentially reverse immune resistance.11 17 Nevertheless, most related studies have concentrated on melanoma, with scarce research in the field of lung cancer therapy. Recently, a proof-of-concept clinical trial conducted by a team of researchers in Korea has demonstrated the potential benefits of FMT in a clinical setting other than melanoma, giving us confidence to carry out our study.12
Our study is a single-arm clinical trial, which has the limitations of a restricted sample size and no control group. So, the conclusions may not be convincing. At the same time, some information derived from a single-arm trial, such as QoL and efficacy predictive biomarkers, was relatively limited. Though the results of randomised controlled trials are more convincing, they demand significant investments of human, material and financial resources.18 On this basis, randomised phase II trials seem to be an effective research method, which requires a relatively small sample size compared with traditional randomised controlled trials, and the inclusion of a control group makes it more scientifically rigorous than a single-arm study.19
Ethics statements
Patient consent for publication
Acknowledgments
We would like to express our sincere appreciation to all the participants.
References
Footnotes
YW and LQ are joint first authors.
YW and LQ contributed equally.
Contributors YW and LQ are responsible for the article’s concept and writing. QG, DL and DQ are responsible for clinical treatment. QG, LQ, YW and XW are responsible for data collection and compilation. HJ and QG coordinate all the work. QG is the guarantor, reviewed the entire study design and the draft. All authors review and approve the final manuscript.
Funding This study was supported by the 2022 Clinical Research project of Changzhou Medical Center, Nanjing Medical University (CMCC202201), 2022 Changzhou 8th Batch of Science and Technology Project (Applied Basic Research) (CJ20220086).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.