Article Text
Abstract
Objectives Hormonal and age-related differences in body composition require tailored approaches for predicting new-onset type 2 diabetes (NODM). Previous studies lacked in-depth stratified analyses. We investigate sex- and age-specific body composition indices associated with NODM.
Design and setting A retrospective, nationwide, population-based cohort study.
Participants We analysed 4 058 891 adults who underwent a health examination in the year 2009 and 10-year follow-up data from the National Health Insurance Service.
Outcome measure NODM risk stratified by sex and age groups in 20-year intervals according to quartiles or per 1 SD increase in body mass index (BMI), waist circumference (WC), waist-height ratio (WHtR), visceral adiposity index (VAI), a body shape index (ABSI) and weight-adjusted waist index (WWI).
Results Among the total subjects, 625 715 individuals (15.4%) developed NODM during median 10-year follow-up. The fourth quartile of WHtR showed the highest HR for NODM compared with the first quartile among various indices across the entire population (HR 2.54, 95% CI 2.52 to 2.57). In men, WHtR consistently exhibited the strongest association with NODM across all age groups in analysis based on 1 SD increase; ages 20–39 years (HR 1.54, 95% CI 1.53 to 1.55), ages 40–59 years (HR 1.39, 95% CI 1.38 to 1.39), ages 60–79 years (HR 1.23, 95% CI 1.22 to 1.24). In women, the most relevant body composition index for NODM varied by age group; BMI for ages 20–39 years (HR 1.48, 95%CI 1.47 to 1.49), WHtR for ages 40–59 years (HR 1.46, 95% CI 1.45 to 1.47) and WC for ages 60–79 years (HR 1.23, 95%CI 1.22 to 1.24).
Conclusion WHtR was the strongest predictor of NODM in men across all ages, while the relevant indices varied by age group in women. These findings highlight the need for sex- and age-specific body composition assessments in predicting NODM risk.
- Body Mass Index
- Diabetes Mellitus, Type 2
- Preventive Health Services
- Obesity
Data availability statement
Data are available upon reasonable request. Study protocol, raw data that support the findings are available from the corresponding author upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATION OF THIS STUDY
The large-scale study using the National Health Insurance Service database represents almost the entire Korean population with 10-year longitudinal follow-up results.
Sex- and age-group-stratified risk of new-onset type 2 diabetes mellitus was estimated.
Due to the lack of laboratory data such as glycated haemoglobin or oral glucose tolerance test results, the severity of diseases could not be evaluated.
The study focused on the impact of the baseline body composition index on long-term outcomes but did not account for changes in body composition during the follow-up period.
Introduction
The number of patients diagnosed with type 2 diabetes mellitus (T2DM) has been rapidly growing. In 2021, 540 million patients were diagnosed with T2DM worldwide, which is fivefold the number of diabetes cases in 1980.1 Similarly, a Korean epidemiological study reported that in 2020, 16.7% of adults ≥30 years of old had T2DM, compared with 13.8% in 2018.2 Because DM is associated with cerebro-cardiovascular complications and mortality, discovering a screening tool for T2DM is important for early diagnosis. Body composition indices provide simple, rapid and inexpensive ways to evaluate risks of metabolic disease by simply measuring anthropometric parameters.
Since its development in 1932, body mass index (BMI) has been a cornerstone for measuring obesity.3 However, in 2007, research from the Framingham Heart study revealed a stronger impact of visceral adipose tissue (VAT) on metabolic disease compared with subcutaneous adipose tissue (SAT).4 VAT releases free fatty acids (FFAs), contributing to insulin resistance, whereas SAT serves as a buffer of FFAs.5 With the recognition of VAT’s significance, alternative body composition indices, such as waist circumference (WC), waist-hip ratio (WHR) and waist-height ratio (WHtR), have been developed to highlight the hazardous metabolic effect of VAT.6 Later, more complex indices such as a body shape index (ABSI) and visceral adiposity index (VAI) were introduced, showing strong links to cardiometabolic risk and insulin sensitivity.7 8 The ABSI is statistically constructed metric designed to capture the metabolic impact of WC independently of height, weight and BMI, using linear least-square regression.7 The VAI is developed based on the fact that the harmful effect of visceral fat is related to high triglyceride (TG) and low high-density lipoprotein cholesterol (HDL-C) levels.9 In lipid metabolism, increased VAT leads to hypertriglyceridemia, which promotes lipoprotein remodelling and accelerates HDL-chol catabolism.5 By incorporating the detrimental effect of a high TG/HDL-C ratio along with increased WC, VAI was formulated and has demonstrated its ability to predict metabolic disease in several studies. Furthermore, recognising the metabolic risks of sarcopenia, researchers developed the weight-adjusted waist index (WWI) to reflect low muscle mass.10 WWI was created by standardising WC with body weight. In several studies, WWI has shown a positive association with VAT and a negative association with appendicular skeletal muscle mass, making it a distinctive parameter for effectively capturing reduction in muscle mass, unlike other existing body composition.11
In previous studies, some indices were investigated in terms of new-onset T2DM (NODM) development12; however, a comprehensive comparison of all six indices, including tailored age- and sex-related risk stratification has not been conducted. However, the increasing prevalence of sarcopenia exerts a significant impact on metabolic risk as people age. In addition, changes in body composition also differ between the sexes. Menopause causes 15–20% of additional abdominal fat accumulation, and decreasing testosterone in men induces muscle mass reduction.13 Therefore, we hypothesised that different body composition indices would be associated with NODM between a young muscular population and a sarcopenic older population, or between men and women. In the present study, body composition indices regarding the prediction of NODM were compared in different age and sex groups using 10-year longitudinal national comprehensive cohort data.
Methods
Data source and study population
In the present study, claims data from the National Health Insurance Service (NHIS) database from 1 January 2004 to 31 December 2019 were used. Korea has a universal single-payer national health system, and the NHIS maintains national records of all covered inpatient and outpatient visits, procedures and prescriptions. The International Classification of Disease version 10 (ICD-10) codes were used to identify medical history and new onset of disease. The study included men and women between 20 and 80 years of age who underwent a health examination between 1 January 2009 and 31 December 2009 and satisfied the following criteria: no history of DM (E10–14), liver disease (K70–77), pancreas disease (K85–87), pancreatectomy (E89.1) or cancer (C00–97) within 5 years or who used a glucocorticoid within 1 year from the date of health check-up at the time of data collection, all of which might affect onset of DM as a secondary cause. Participants with fasting blood glucose ≥126 mg/dL at the health examination in 2009, participants with missing and outlier values for height, weight, WC, TG and HDL-C for the calculation of anthropometric index, and subjects who were deceased at baseline were excluded. Ultimately, a total of 4 058 891 patients were included in the analysis. For the examination of body composition indices reflecting age- and sex-dependent DM risk stratification, we divided the study total population into three groups: young adults (20–39 years), middle-aged (40–59 years) and elderly (60–79 years). Individuals in their 40s and 50s often experience changes in body composition due to childbirth and the perimenopausal period, while those in their 60s and 70s typically face a loss of muscle mass and strength.14 The detailed study flow is shown in online supplemental figure 1. The Korea University Institutional Review Board approved this study (No. 2022GR0041) which was conducted in accordance with the Declaration of Helsinki of the World Medical Association. We were permitted to use the NHIS data by the NHIS review committee (NHIS-2022-1-704). The need for informed consent was waived because anonymous and de-identified information was used for analysis.
Supplemental material
Definition of body composition index and variables
The characteristics of NHIS databases were presented in previous studies.15 Among various body composition parameters, six body composition indices were selected for the present study: BMI, WC, WHtR, VAI, ABSI and WWI, which have been widely studied in large cohorts. Because NHIS has no data on the hip circumference, the effect of WHR could not be analysed in this study. The body composition indices were calculated with the following equations: BMI as weight/height,2 3 WHtR as WC/height,4 6 ABSI as 1000×WC × weight−2/3 × height5/6,16 VAI as WC/[39.68 + (1.88×BMI)] × TG/1.03×1.31/HDL (for men) and WC/[36.58 + (1.89×BMI)] × TG/0.81×1.52/HDL (for women),8 and WWI as WC/√weight.17 Age, sex, residential area and income percentile were obtained from the insurance eligibility database. Smoking status, alcohol consumption, regular exercise, height, weight, family history of diabetes, TG and HDL-C were obtained from the health screening examination database. An area with a population >0.3 million was classified as urban. Alcohol consumption was classified as none, moderate (1–14 cups per week for men, 1–7 cups per week for women), heavy (≥15 cups per week for men, ≥8 cups per week for women) or unknown. Regular exercise was defined as none, regular (vigorous activity for ≥3 days or moderate-intensity activity for ≥5 days), irregular (other activities including walking) or unknown.
Study outcomes
The study outcome was the incidence of NODM defined by searching for either ICD-10 codes E11–14, usage of an oral hypoglycaemic agent or a fasting plasma glucose level of ≥126 mg/dL.18 The duration of follow-up was defined as the time interval between the health examination date and the date of the study outcome, death or censoring or the end of the study period (31 December 2019). We evaluated the relationship between six body composition indices—BMI, WC, WHtR, VAI, ABSI and WWI—and the onset of diabetes mellitus (NODM) over a 10-year period. These indices were compared based on their association with NODM across different 20-year age intervals and between sexes.
Statistical analysis
The baseline characteristics of the study population are presented as mean±SD for continuous variables and number (percentage) for categorical variables. The ANOVA and χ2 test were used to compare the characteristics between men and women with different age groups and between subjects with and without T2DM. The incidence rate of NODM was calculated by dividing the number of subjects diagnosed with NODM by person-years. Cox proportional hazards regression was used to identify the association between anthropometric indices and NODM. Calculated HRs and 95% CIs were estimated based on the quartiles and 1 SD of each body composition index. To account for additional potential confounding factors at the baseline health examination, age, sex, smoking status, alcohol consumption, regular exercise, family history of diabetes, residential area and income percentile were adjusted (models 1–5). Additional adjustment with systolic blood pressure, diastolic blood pressure, TG and HDL-C was presented in online supplemental material (model 6). Not only does the VAI formula contain TG and HDL-C, but also the main purpose of our study is to estimate NODM risk by measuring body composition without laboratory tests. Hence, we presented results of models 1 through 5 in the main findings. P value <0.05 was considered statistically significant. All analyses were performed using SAS V.9.4 (SAS Institute Inc., Cary, NC, USA).
Patient and public involvement
Since this study used publicly available datasets, there was no direct patient or public involvement in the planning, execution or analysis steps. Nevertheless, we understand the value of integrating patients and public insights into research. We plan to disseminate our findings to patients and the public as valuable information for T2DM prevention.
Results
Baseline characteristics of the study population and 10-year cumulative incidence of NODM
For the 10-year follow-up period, 625 715 subjects were diagnosed with NODM (15.4% of the total population). The mean age of the study population was 41.9±12.2 years and 60.7% of participants were men. The incidence rate of NODM for the 10-year period was 16.2% in men and 13.3% in women. When age was stratified by two decades, 10-year T2DM incidence in both men and women markedly increased almost twofold in the middle-aged group (40–59 years) compared with the young-adult group (20–39 years) (men: 14.0% in 20–39 years age group, 27.4% in 40–59 years age group; women: 10.4% in 20–39 years age group, 22.7% in 40–59 years age group; figure 1). In the elderly group, 32.9% of men and 30.7% of women progressed to T2DM during the 10-year follow-up period. The detailed baseline characteristics of men and women with age groups are presented in table 1. In obesity classification based on BMI, the percentage of obese men decreases at the ages of 60–79 years, while the percentage of obese women increases at the ages of 60–79 years. A comparison of baseline characteristics of patients with or without incidental NODM is shown in online supplemental table 1.
10-year incidence of T2DM stratified by 10-year age groups in (A) men and (B) women.
Baseline characteristics of patients with or without incident T2DM
Association of the quartiles of body composition indices and NODM incidence across different 20-year age intervals and between sexes
Of the various body composition indices, WHtR exhibited the highest HR for the NODM in the fourth quartile group compared with the first quartile in all models of Cox regression. NODM HR of WHtR in the fully adjusted model was 2.54 (95% CI 2.52 to 2.57) followed by WC, BMI, VAI, WWI and ABSI with HRs of 2.54, 2.52, 2.31, 2.19, 1.78 and 1.18, respectively (table 2). When we further adjusted for systolic blood pressure, diastolic blood pressure, TG and HDL, which are directly influenced by anthropometric parameters, WHtR still showed the highest HR for NODM among all body composition indices (online supplemental table 2).
HRs and 95%CI of NODM across quartiles of body composition indices in total population
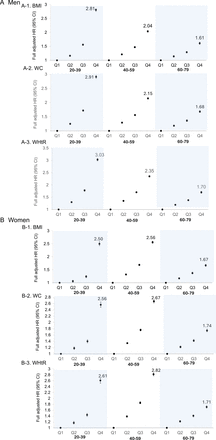
Subgroup analysis was performed to examine the best body composition indices associated with NODM in different sex and 20-year interval age groups. As shown in figure 2A, in men, WHtR had the highest HR for NODM across all age groups compared with other indices. The impact of the body composition indices on NODM was most significant in men aged 20–39 years, showing a gradual reduction in HR with ageing. However, in women, the effect of body composition indices on NODM was the greatest in the middle-aged group (ages 40–59 years), with HR values numerically higher than those in both young-adult women (ages 20–39 years) and elderly women (ages 60–79 years) (figure 2B). Similar to men, the WHtR had the highest HR for NODM in women, except in the elderly group.
HRs of T2DM across quartiles of body composition indices according to 20-year age group in men (A) and women (B). BMI, body mass index; T2DM, type 2 diabetes mellitus; WC, waist circumference; WHtR, waist-height ratio.
Association of 1 SD increment of body composition indices with NODM across different 20-year age interval groups and sex groups
In the regression analysis based on quartiles of indices, HRs of NODM were markedly increased in the fourth quartile of indices compared with risk increments observed in the second and third quartiles. To avoid overestimating the association between NODM and the body composition indices, which might occur due to high correlations observed in the fourth quartile, we also evaluated the association between NODM and a 1 SD increase in each index. The relative risk of NODM with a 1 SD increase of body composition indices in each sex and age group is shown in figure 3A,B. In men, WHtR had the highest HR across all age groups with 54% increased risk in young adults, 39% in middle-aged and 23% in the elderly per 1 SD increase, showing a sequential decrease in HR values as age increased (figure 3A and online supplemental table 3). Conversely, in women, differential indices showed the highest risk of NODM with a 1 SD increase in each age group; BMI had the highest HR in young adults, WHtR in middle-aged subjects and WC in the elderly. The association of indices related to WC and WHtR remained significant up to middle age but diminished in older age groups (figure 3B and online supplemental table 3).
HR of new-onset T2DM in a fully adjusted model according to a 1 SD increase of anthropometric indices stratified by 20-year age groups in men (A) and women (B). ABSI, a body shape index; BMI, body mass index; NOFM, new-onset T2DM; T2DM, type 2 diabetes mellitus; VAI, visceral adiposity index; WC, waist circumference; WHtR, waist-height ratio; WWI, weight-adjusted waist index.
Discussion
The present research included a nationwide cohort database and found that a distinct body composition index adequately predicted the risk of 10-year NODM in different age and sex groups. The incidence of NODM increased twofold from the young-adult group to the middle-aged group. WHtR was strongly associated with NODM in men across all age groups. In women, BMI in young adults (ages 20–30 years), WHtR in middle-aged (ages 40–50 years) and WC in the elderly (ages 60–70 years) showed a strong association with NODM based on 1 SD increase of each index. In men, the influence of body composition indices on NODM was the greatest in the young-adult group, with a sequential decrease as age increased. In women, the significant effect of body composition indices was maintained in the middle-aged group but showed a decreased impact in the elderly group. Although the predictive power of the body composition index for NODM was not generally high, we emphasise that consistent application of BMI for assessing metabolic risk in all age and sex groups should be avoided.
Since Bray and Popkin warned of a crisis of diabetes incidence in 2014,19 the prevalence of T2DM rapidly grew worldwide increasing the number of individuals with T2DM every year. In addition to traditional risk factors for T2DM such as obesity, age, ethnicity and familial history of DM, frequent exposure to highly processed food and a sedentary lifestyle warrants periodic screening for T2DM regardless of age. In the Laiteerapong study group, immediate glycaemic control after the onset of DM was shown important for the reduction of vascular complications and mortality.20 The legacy effect evidenced in this study emphasises the significance of early detection and management of DM. BMI has been used as an easy and rapid method to screen DM risk and evaluate the degree of obesity. To compensate for the limitations of BMI in discriminating between visceral and subcutaneous fat, WC, WHtR and ABSI were suggested.4 6 7 Laboratory data such as TG and HDL-C were adopted in the VAI equation,8 and the development of WWI was used to discriminate between muscle and fat mass.11
In several studies, a comparison of new indices to BMI for DM screening has been reported. In a Chinese study that included 8121 subjects aged 35–60 years, WHtR was reported as more effective than BMI or WC for T2DM prediction.21 In the meta-analysis of 31 studies, WHtR was significantly associated with T2DM compared with BMI in both men and women.22 In another meta-analysis including 22 prospective studies, WHtR and WC showed similar predictability for DM and were superior to BMI.23 Furthermore, Saberi-Karimian et al reported that WC predicted T2DM development better than BMI, ABSI, WHtR and WHR in their prospective study with 9354 subjects.24 In a study that included 3461 Chinese participants, VAI was reported as an effective index for DM prediction compared with WC, WHtR and BMI.25 Differences in study results might be due to the heterogeneity of the study populations and characteristics. Unlike previous studies, risk stratification was performed in the present study with subjects of different ages and sex because they may have different metabolic risks and body composition.
Based on the study results, in women, age had a significant effect on body composition index that is most relevant to incidental DM. Women experience significant changes in hormones and body composition during pregnancy, childbirth and menopause. Cho et al showed weight gain in women during the perinatal period mostly resulted from visceral fat deposition,26 and in other studies, the number of parity was positively associated with abdominal obesity.27 Furthermore, in previous research, fat mass volume doubled, and lean mass decreased at the start of menopause with the reduction in oestrogen level.27 In several studies, body fat distribution was shown to change from the extremities to the trunk during the perimenopausal period.28 These undesirable body composition changes are strongly associated with cardiometabolic disease risks, increasing incidence of DM, dyslipidaemia and cardiovascular disease in postmenopausal women.29 Therefore, the significant increase of DM incidence in women in the 40–59 years age group compared with the 20–39 years age group in the present study was likely because women undergo a decrease in oestrogen in their mid-to-late 40s and 50s. Regarding the body composition index associated with DM, we demonstrated that every 1 SD increase in BMI exhibited the strongest HR for NODM in the young women; however, in the middle-aged women, that of WHtR showed the highest HR for NODM. This result may be due to visceral fat accumulation and undesirable body compositional changes after women experience pregnancy and delivery through their 40s and menopause in their 50s.
However, in men, the WHtR had the closest relationship with NODM across all age groups. In numerous literatures, WHtR was shown superior to BMI for DM prediction.23 25 The reason why WHtR is better than BMI for DM prediction is due to the inclusion of visceral obesity in the WHtR index. Visceral adiposity induces insulin resistance, dysregulation of adipokines and malfunction of adipocytes, resulting in NODM.30 However, the reason WHtR is better than WC for NODM prediction remains unknown although several theories have been suggested. A plausible explanation is short stature, which is largely due to genetic factors and might affect visceral obesity, insulin resistance, and vulnerability to metabolic diseases.31 In addition, because WHtR adjusts for the effect of height on WC, it reflects a different body fat distribution and muscle mass based on individual height.13
Notably, in the young-adult group, the BMI of women and WHtR of men had the highest HRs for NODM with a 1 SD increase, indicating body composition associated with NODM differs between sexes. Women store excess fat first in a metabolically healthy fat region such as subcutaneous fat and lower extremities and then in VAT, whereas men predominantly accumulate fat more rapidly in VAT.32 For women, the importance of VAT starts to appear in the postpartum and perimenopausal period. The present study showed that WHtR was significantly associated with the risk of NODM, especially in young-adult men with a sequential decrease with ageing; however, in women, the clinically significant effect of WHtR for NODM was obvious around the perimenopausal period. Considering the emerging trend in early-onset DM leading to higher incidence of microvascular and macrovascular complications, intervention that reverses or reduces abdominal obesity, especially in young-adult men and perimenopausal women, is critical.
The present study had several limitations. Because claims data from NHIS were analysed, subjects were included and excluded based on ICD-10 codes that clinicians reported to the insurance service. Although we attempted to exclude subjects with secondary causes of DM, including related diseases or medication, some undefined cases may have been inadvertently included or excluded. Due to the absence of data on the dose and duration of glucocorticoid use, we exclude all individuals who prescribed glucocorticoid within 1 year. Also, patients using other medications that could affect the development of DM, such as statin, antipsychotics or antiretrovirals, were not excluded. Due to the absence of information on eating habits in our database, we were unable to adjust the effect of diet habits in analysis. Furthermore, due to the lack of laboratory data such as glycated haemoglobin or oral glucose tolerance test results, the severity of diseases could not be evaluated. In addition, any causal relationship could not be evaluated due to the intrinsic restriction of observational studies. Additionally, although we performed receiver operating characteristics analysis to compare the predictive power of each body composition index, the onset of DM in the elderly tend to be multifactorial beyond the effect of body composition, resulting in a decrease in the area under the curve with increasing age. This reduction in predictive power was insufficient to compare the superiority of the indices, so we did not proceed to the pairwise comparison of indices. Finally, because only data of Koreans were included, age- and sex-stratified DM risk should be evaluated in different ethnicities. However, this is the first and most recent large-scale study in which significant body composition indices for NODM were compared using a 10-year longitudinal follow-up NHIS database representing almost the entire Korean population. Subjects with probable secondary causes for DM were excluded, and multiple variables were adjusted that could affect the onset of DM to examine the effect of body composition indices on NODM incidence.
Conclusion
WHtR in men of all age groups, BMI, WHtR and WC in young-adult, middle-aged and elderly women, respectively, had the strongest association with the 10-year incidence of NODM in the Korean population. Applying an age- and sex-specific body composition index is important to appropriately screen for the risk of NODM. Because the incidence of NODM significantly increased in the middle-aged group in both men and women, active surveillance for NODM risk by measuring WC and height is necessary for effective prevention.
Data availability statement
Data are available upon reasonable request. Study protocol, raw data that support the findings are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by The Korea University Institutional Review Board (No. 2022GR0041). The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association. The need for informed consent was waived because anonymous and de-identified information was used for analysis.
Acknowledgments
This research was supported and funded by the Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL) grant (O2208141).
References
Footnotes
MJP and MK contributed equally.
Contributors Conceptualisation and design, data interpretation and writing—original draft: HJY, MJP and MK. Data acquisition and analysis: MK. Writing—review and editing: AJ, SYJ, ES, KMC and SHB. HJY is the guarantor and takes responsibility for the overall content.
Funding This research was funded by Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL) grant (O2208141) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF), South Korea, funded by the Ministry of Education (NRF-2021R1A2C2008792). These funding sources had no role in the study design, data analysis, or writing. We thank the staffs at the Big Data Steering Department of the National Health Insurance Service and the subjects in this study.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.