Article Text
Abstract
Introduction Given that axillary lymph node dissection (ALND) may not contribute to local control or survival and could lead to increased arm morbidity, axillary de-escalation procedures have replaced ALND in patients achieving axillary pathologic complete response (apCR) after neoadjuvant systemic therapy (NST). However, the application of targeted lymph node biopsy, one of the de-escalation procedures, remains limited due to a lack of long-term follow-up studies.
Methods and analysis This prospective, single-arm, open-label, non-inferiority, single-centre phase II trial targets breast cancer patients initially diagnosed with axillary metastasis who achieved apCR after NST. The study aims to validate the oncological safety of stained region lymph node biopsy (SrLNB) procedure. SrLNB is a novel de-escalation axillary surgery, which was developed and tested in our preliminary study. The primary endpoint of this trial is the 3-year invasive disease-free survival (iDFS). Secondary endpoints include local-regional recurrence, incidence of breast cancer-related lymphoedema and patient-reported outcomes. The 3-year iDFS in patients undergoing ALND is expected to be approximately 90%, with a non-inferiority margin of 10%, a significance level of 0.05, power of 0.8 and a loss-to-follow-up rate of 10%. The planned enrolment is 92 patients. The trial was initiated on 11 September 2023, with the first patient enrolled on 25 September 2023, and is scheduled to end in 2026.
Ethics and dissemination The trial protocol received approval from the Human Research Ethics Committee of The First Affiliated Hospital with Nanjing Medical University in May 2023 (No. 2023-SR-169). All participants will provide informed consent. The study results will be disseminated through international peer-reviewed scientific journals, presentations at international scientific conferences and public lectures.
Trial registration number NCT05939830.
- Breast surgery
- Breast tumours
- Clinical Trial
- Patient Reported Outcome Measures
- Quality of Life
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The stained region lymph node biopsy (SrLNB) procedure is economical and easy to conduct with the use of carbon tattooing and can be considered a further de-escalation of the standardised sentinel lymph node biopsy procedure.
The SrLNB study places greater emphasis on arm morbidity and quality of life with multiple objective and subjective measurements.
The small sample size and the single-centre design of the study may lead to some limitations in the extrapolation of the results.
Introduction
Axillary lymph node dissection (ALND) has been replaced by sentinel lymph node biopsy (SLNB) for cN0 patients (clinically axillary lymph node-negative), based on randomised studies showing that ALND does not improve oncological outcomes but is associated with substantial upper limb morbidity.1 Axillary de-escalation surgery can reduce surgical morbidity rate (ie, lymphoedema, chronic pain and mobility restrictions) and improve patients’ quality of life (QoL).
For patients with cN+ at the time of diagnosis, neoadjuvant systemic therapy (NST) is often the option. With great advances in NST, axillary pathological complete response (apCR) has been observed in 13%–60%2 of patients with axillary metastases at the time of diagnosis in different molecular subtypes, especially in 59% of HER2-positive patients and 48% of triple-negative breast cancer patients (TNBC).2 For patients with axillary nodes remaining clinically positive (ycN+) after NST, ALND of I–II levels remains the standard management, whereas in patients with lymph nodes downstaged to negative (ycN0), the necessity of conventional ALND has been questioned. To date, ALND has been replaced by de-escalation procedures like standardised SLNB or targeted axillary dissection (TAD).
To replace ALND with de-escalation procedures, one of the key points lies in the false negative rate (FNR). ACOSOG Z1071,3 SN FNAC4 and SENTINA5 studies modified SLNB by use of dual tracing agents and removal of ≥3 sentinel nodes to make FNR below the threshold value of 10%. In the TAD procedure6, suspicious lymph nodes marked by various methods before NST will be biopsied (targeted lymph node biopsy, TLNB) along with SLNB, which can reduce FNR to 2%. A study by Donker et al7 proposed the MARI procedure, marking the axillary lymph node with radioactive iodine (125I) seeds, where only TLNB were dissected without SLNB, with an acceptable FNR of 7%, thus TLNB alone can be considered as an option for de-escalation procedure. In addition, various methods of marking and localising metastatic lymph nodes have been developed, such as iodine-125 seed,8 carbon tattooing9 and Magseed.10 However, the optimal marking and localising methods for TLNB are under discussion due to a lack of comparative data. Additionally, the use restriction of radioactive particles makes standardised SLNB, TAD and relevant TLNB unrealistic in China and some other countries.
Based on prior studies and clinical practices in China, we developed stained region lymph node biopsy (SrLNB) as a modification of TLNB. When conducting fine-needle aspiration (FNA), a suspension of carbon nanoparticles (0.1–0.2 mL, LUMMY, Chongqing, China) will be injected into the cortex of the biopsied nodes immediately. SrLNB requires removing all lymph nodes within the stained region and retrieving abnormally palpated lymph nodes near the stained region. Data from our previous research indicated that SrLNB has a high detection rate and a FNR below the threshold value (10%).11 This suggests good diagnostic performance and the potential to downstage axillary surgery. The SrLNB procedure offers several benefits with the use of carbon tattooing: eliminating the need for a second localisation prior to surgery, free of radioactive particles, allowing for the completion of axillary surgery at one time, and providing a long identifiable period (up to half a year) for black-stained lymph nodes by carbon nanoparticles. SrLNB also has the drawback of increased axillary tissue trauma due to the need for axillary dissection to visually identify the marked node. Additionally, it may cause black staining of axillary tissue and dye migration, although these risks are minimised by controlling the amount of carbon nanoparticles. Despite these limitations, SrLNB remains a highly promising approach for axillary de-escalation surgery.
Nevertheless, oncology outcomes, including local control and survival, must be considered when only de-escalation axillary surgery such as SLNB, TAD, TLNB or SrLNB is performed without ALND. To date, several retrospective and prospective studies12–17 have reported prognostic data for standardised SLNB or TAD, while robust evidence regarding the oncological prognosis of TLNB remains limited. A review reported a low axillary recurrence of 0%–3.4%,18 and a meta-analysis19 reported a pooled 5-year disease-free survival (DFS) of 86% in the SLNB group, with no significant difference to the ALND group (p=0.24). In the study of Wu et al,17 152 patients who received TAD had a 3-year recurrence-free survival of 100%, with no significant difference from the ALND group (p=0.254). The SenTa study16 also reported a 3-year invasive DFS (iDFS) of 91.2% in 119 patients, and only TAD without ALND was not associated with the risk of recurrence or death (p=0.69, p=0.91, respectively). However, this was an observational study without treatment intervention, and axillary metastasis was clinically diagnosed without pathological examination.
Regarding TLNB, there is very little high-quality evidence of long-term prognostic outcome, limiting its clinical application. Therefore, this study is designed to assess the oncological safety of SrLNB procedures. Breast cancer patients with pathologically confirmed axillary metastasis (pN+) who are clinically downstaged to negative (ycN0) after NST will be enrolled. Among them, ypN0 patients identified by SrLNB will not undergo ALND and will subsequently receive regional lymph node radiotherapy (RNI) including the axilla, while ypN+patients will be excluded and undergo ALND. The oncological safety of SrLNB will be primarily evaluated by a 3-year follow-up of iDFS and local-regional recurrence rate (LRR). This will also add evidence to the oncological safety of TLNB. Additional focus will be given to the incidence of breast cancer-related lymphoedema (BCRL) and QoL, which will provide a subjective perspective to assess the potential of SrLNB in reducing arm morbidity and QoL.
Methods and analysis
Study design and setting
The SrLNB is a prospective, single-arm, single-centre, open-label phase II clinical trial. It is currently being conducted by the First Affiliated Hospital with Nanjing Medical University in China. Women with FNA-confirmed lymph node metastases, downstaged to ypN0 after NST and confirmed by SrLNB, will be enrolled and will not undergo ALND. RNI covering the axilla (including the supraclavicular region and axillary regions I, II and III) will be administered, followed by a 3-year follow-up. Prophylactic irradiation of the internal mammary node (IMN) drainage area will be added when necessary. It is expected that the 3-year iDFS in patients undergoing ALND is about 90%, and a non-inferiority margin value of 10%, with a significance level of 0.05 and power of 0.8, loss to follow-up rate of 10%, the planned enrolment is 92 patients. Enrolment is expected to be complete within 2 years, with follow-up scheduled to complete in 2026.
The primary aim of this study is to assess the 3-year iDFS of ypN0 patients receiving SrLNB without ALND. Secondary endpoints include the LRR, BCRL rate, QoL and patient-reported arm morbidity. BCRL will be assessed by relative volume change (RVC) and single-frequency bioimpedance analysis (SFBIA). QoL will be assessed by FACT-B (V.4.0) and patient-reported arm morbidity will be assessed by Quick Disability of the Arm, Shoulder and Hand (QuickDASH) questionnaire. This study evaluates the local control and oncological outcomes of the SrLNB procedure, with additional focus on arm morbidities and QoL.
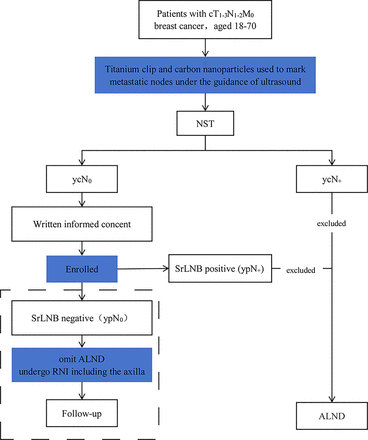
The study was reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) checklist.20 The detailed flow chart of the study is shown in figure 1. The trial schedule based on the SPIRIT checklist is presented in table 1.
Schedule of enrolment, interventions, and follow-up for each participant
Supplemental material
Flow chart of SrLNB trial. ALND, axillary lymph node dissection; cTNM, clinical classification of tumor, node and metastasis; NST, neoadjuvant systemic therapy; RNI, regional lymph node radiotherapy; SrLNB, Stained Region Lymph Node Biopsy; ypN0, pathologically assessed lymph node-negative after NST.
Trial participants and inclusion/exclusion criteria
Inclusion criteria
Patients who meet the following criteria are eligible for inclusion:
Females, aged between 18 and 70.
Histologically confirmed invasive breast cancer (regardless of pathological type) with a clinical stage of cT1–3.
Pathologically confirmed positive axillary lymph nodes with a clinical stage of N1–3.
Received a full course of NST (including neoadjuvant chemotherapy, neoadjuvant targeted therapy, neoadjuvant immunotherapy).
Positive axillary lymph nodes successfully stained by carbon nanoparticle suspension.
All patients are required to undergo immunohistochemical staining (IHC) for oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), Ki-67 proliferation index and further fluorescence in situ hybridisation should be performed in HER2 2+cases.
Metastatic axillary lymph nodes downstaged to clinically negative after NST (ycN0) assessed by ultrasound and MRI.
ECOG score of 0–1.
Patients voluntarily participated in this study and signed the informed consent form (see online supplemental material).
Exclusion criteria
Patients will be excluded on any of the following grounds:
Bilateral breast cancer.
Breast cancer during lactation or pregnancy.
Presence of distant metastases confirmed by physical examination or imaging examination.
History of a malignant tumour.
History of previous surgery on the affected axilla, or history of surgery affecting the function of the upper extremity.
History of radiation therapy to the breast or chest.
Positive surgical margins for breast-conserving surgery/mastectomy.
Positive results of intraoperative frozen-section evaluation (including isolated tumour cells and micrometastases) for SrLNB (ypN+).
Inability to complete the full course of adjuvant therapy as prescribed.
Aspartate transaminase or alanine transaminase ≥1.5 times the upper limit of normal, alkaline phosphatase ≥2.5 times the upper limit of normal, total bile ≥1.5 times the upper limit of normal, serum creatinine ≥1.5 times the upper limit of normal; left ventricular ejection fractions <50% on cardiac ultrasound; severe coagulation dysfunction, serious systemic disease or uncontrolled infection.
Lack of personal freedom and independent civil capacity.
Presence of mental disorders, addictions and those deemed ineligible by the investigator.
Sample size
This study is a single-arm objective performance criteria study, with the 3-year iDFS of ypN0 patients receiving standard treatment (ALND and RNI) as the target, which is expected to be 90% as described in previous studies.12–19 21–24 A non-inferiority test will be used to evaluate the 3-year iDFS with the margin value of 10%, with a significance level of 0.05 and power of 0.8. Assuming a 10% rate of loss to follow-up, the required sample size is 92 (calculated by PASS V.15.0 software). In light of this, a 3-year iDFS>80% for enrolled patients who underwent SrLNB is considered non-inferior to ALND in terms of oncology safety, and SrLNB followed by RNI including the axilla is non-inferior to the standard of care.
Intervention
Neoadjuvant systemic therapy
All enrolled patients will receive NST, which will be determined by the molecular subtype as clarified by the status of ER, PR and HER2. ER and PR positive are both defined as ≥1% of the invasive tumour cells staining positive via IHC. HER2 positive is defined as HER2 3+ and HER2 2+ by IHC with the presence of HER2 amplification by FISH (ISH+). The chemotherapy regimen combines sequential paclitaxel and anthracycline, with trastuzumab and/or pertuzumab added for HER2-positive patients. The course of NST typically spans 6–8 cycles and will be individually adjusted.
Breast surgery
Breast-conserving surgery or mastectomy is selected based on tumour characteristics and patient preference. Patients who undergo breast-conserving surgery will receive whole-breast irradiation (WBI) afterwards.
Axillary surgery
SrLNB procedure
Before the NST, the most abnormal lymph node will be selected for FNA. At the same time, carbon nanoparticle suspension (0.5 mL containing 25 mg; LUMMY, Chongqing Lummy Pharmaceutical Co. Ltd, China) will be diluted (concentration: 0.05 mL per 0.15 mL saline) and injected into the cortex of the biopsied lymph node under ultrasound guidance to ensure that the biopsied lymph node is accurately stained and marked. Only the most abnormal node will be stained, unless multiple abnormal lymph nodes are distributed across a wide area, in which case the closest and most distal nodes within the affected region will be stained to delineate the surgical field boundaries. Lymph nodes will turn black after staining. During surgery, SrLNB involves the removal of the stained lymph node (or the stained region in cases of widely distributed multiple abnormal lymph nodes), along with any abnormally palpated lymph nodes near the stained node. Those with negative results of intraoperative pathology will be enrolled, while those with macrometastases, micrometastases or isolated tumour cells will be excluded from this study.
Axillary lymph node dissection
Enrolled patients will be exempted from ALND, which includes axillary lymph nodes in regions I and II. However, patients with residual axillary disease will routinely undergo ALND.
Radiotherapy
All patients enrolled will undergo WBI or postmastectomy radiotherapy (PMRT) according to breast surgery and RNI including the axilla, regardless of treatment response to systemic therapy. Part of the patients will receive prophylactic irradiation of the IMN drainage area when necessary.
Those undergoing breast-conserving surgery will receive WBI, with a boost to the tumour bed delivered sequentially immediately after. The prescribed dose for conventional fractionated irradiation is 50 Gy in 25 fractions with a single dose of 2 Gy, administered 5 times a week. The dose to the tumour bed is 60 Gy in 25 fractions with a single dose of 2.4 Gy. Alternatively, when a hypofractionated regimen is applied, the dose will be 43.5 Gy in 15 fractions of 2.9 Gy over 3 weeks, and the dose to the tumour bed will be 52.5 Gy in 18 fractions of 2.9 Gy.
PMRT is defined as radiotherapy to the chest wall for those undergoing mastectomy. The dose is 50 Gy/25 F (50 Gy in 25 fractions) for conventional fractionated irradiation or 43.5 Gy/15 F for hypofractionated irradiation.
RNI including the axilla covers the supraclavicular region and the entire axillary lymphatic drainage area (region I, II, and III), with a dose of 50 Gy/25 F.
IMN is added if any of the following criteria are met: (1) ≥4 positive axillary lymph nodes; (2) primary tumour located centrally or in the medial quadrant of the breast, or posterior to the nipple and (3) imaging-diagnosed suspicious metastasis of IMN or pathologically confirmed metastatic IMN.
Adjuvant systemic treatment
Systemic adjuvant therapies will be administered in accordance with local and international guidelines. These therapies include chemotherapy, targeted therapy, endocrine therapy and immunotherapy. Luminal patients mainly receive epirubicin and cyclophosphamide followed by docetaxel (EC-T), combined with endocrine drugs. HER2+ patients mainly receive EC followed by docetaxel, trastuzumab and pertuzumab, or docetaxel, carboplatin, trastuzumab and pertuzumab (TCbHP). To TNBC, docetaxel and carboplatin, followed by EC, with or without pembrolizumab, is the main regimen. The regimen will be individually adjusted based on the actual situation.
Follow-up
Follow-up will commence after surgery, with a 3-year follow-up as the primary endpoint. The schedule for follow-up examinations is as follows:
Physical examination: every 6 months.
Imaging examination: bilateral breast and axillary ultrasound every 6 months; mammography with or without MRI annually for patients who have undergone breast-conserving surgery; chest X-ray or CT, abdominal ultrasound, bone scanning, and head MRI will be conducted annually when necessary.
Objective assessment of BCRL: RVC and SFBIA will be tested and calculated at 1 week, 1, 3, 6, 12, 24 and 36 months postoperation.
Patient-reported questionnaires: FACT-B and QuickDASH will be surveyed at 1 week, 1, 3, 6, 12, 24 and 36 months postoperatively.
Endpoints
Primary endpoint
The primary endpoint of the trial is iDFS, defined as the time interval from the surgery to invasive LRR, distant metastasis, contralateral invasive breast cancer or death from any cause. iDFS will be calculated after a 3-year follow-up. Patients without an event will be censored at the date of the last available assessment.
Secondary endpoints
Local-regional recurrence
This includes both LRR. Local recurrence is defined as recurrence in the ipsilateral breast, chest wall, skin or surgical scar. Regional recurrence is defined as recurrence in the affected lymphatic drainage area, including the axilla, supraclavicular region, subclavicular region and IMN area. LRR will be calculated after a 3-year follow-up.
Breast cancer-related lymphoedema
In our study, two objective methods will be used to assess upper limb lymphoedema. If either criterion is met, BCRL will be diagnosed. In the event of inconsistency, the former results will prevail.
RVC >10% in the affected upper extremity
The volume of the upper limb is calculated based on arm circumference, where 5 points were taken to measure at 10 cm intervals from ulnar styloid to 40 cm away. Five points divide the upper limb into four truncated cones. For each truncated cone, arm circumference of both ends are recorded as C1 and C2, the volume formula is25: V=h(C12+C22+C1 C2)/12π (h is the length, which was 10 cm in the study). The volume of the upper limb is the sum of four truncated cones. The baseline volume of both upper limbs is measured and calculated preoperatively, with the volume of the affected arm recorded as A1 and the unaffected arm as U1. At the time of follow-up, the arm volume is calculated in the same way, with the affected arm recorded as A2 and the unaffected arm as U2. Then, the RVC can be calculated as follows: RVC = (A2/U2)(A1/U1)−1. When RVC>10%, BCRL will be diagnosed.26 Follow-up of RVC will be conducted at 1 week and 1, 3, 6, 12, 18, 24 and 36 months after the operation.
Bioelectrical impedance analysis
The SFBIA ratio is defined as the ratio of the impedance of the unaffected upper limb to the affected one. BCRL is diagnosed when the impedance ratio exceeds the threshold value of the mean±2 SD of healthy controls. The impedance of both arms is measured by the Inbody device at 1 kHz and 5 kHz, and the ratio is calculated. Referring to the study by Liu et al,27 a diagnosis will be made when any one of the following criteria is met: when the affected arm is the dominant hand, its impedance ratio >1.067 at 1 kHz or>1.068 at 5 kHz; when the affected arm is the non-dominant hand, the ratio >1.043 at 1 kHz or >1.044 at 5 kHz suggests oedema.
Quality of life
Patient-reported outcome (PRO) will be reported using the Chinese version of FACT-B (V.4.0). These scales will be used to subjectively assess QoL. They will be surveyed before surgery as a baseline measurement, and follow-up will be conducted at 1 week and 1, 3, 6, 12, 24 and 36 months postoperative.
Patient-reported arm morbidity
Patient-reported arm morbidity will be reported using the Chinese version of QuickDASH questionnaires. They will be surveyed before surgery as a baseline measurement, and follow-up will be conducted at 1 week and 1, 3, 6, 12, 24 and 36 months postoperative.
Data collection and management
An electronic case report form is used for data management and recording. The principal investigator oversees all projects in this trial. Investigators perform data collection, entry, correction and revision. To ensure data accuracy, two data managers will independently perform double entry and proofreading.
Clinical supervisors audit data for completeness, consistency and standardisation. They will challenge any data that are nonsensical or non-standardised. Researchers are expected to respond to and address these challenges promptly, recording the reasons for any modifications. If a participant withdraws consent, no further data will be collected, but existing data will remain anonymised and retained for 3 years. At the study’s conclusion, the principal investigator will submit a final report for review and written approval by the ethics committee.
Statistical analysis
Baseline characteristics, including demographics, pathology features, treatment regimen, BCRL rate and scores of QoL scales, will be described. For continuous variables, data will be presented as mean, SD, or median and IQR. For categorical variables, data will be analysed using the χ2 test or Fisher’s exact test, and presented as totals, percentiles and frequencies. Continuous variables will be analysed using the Student’s t-test or the Mann-Whitney U test.
Kaplan-Meier curves will be established to analyse iDFS and LRR. HRs and 95% CIs are calculated. For iDFS with a single-group target value, a non-inferiority test will be performed: the target value of iDFS for the experimental group is set at 90%, with a non-inferiority cut-off value of 10%, α=0.05, β=0.2 and an estimated 10% loss rate of follow-up. Univariate and multivariate Cox regression models will be used to analyse impact factors on iDFS and LRR. If the LRR events are few, they could be reported as a crude rate. Differences are considered statistically significant with p<0.05.
Data monitoring
An independent data monitoring committee (IDMC) will be established for this study and will undergo review every 6 months. Any serious adverse events (SAEs) that occur during the trial will be documented following the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE V.5.0). Investigators are required to report all adverse reactions, suspected adverse reactions, unexpected adverse reactions and unanticipated events during the trial, in writing to the sponsor and the Ethics Committee within 24 hours of occurrence. Clinical data administrators generate a review report post data cleansing. This report will be discussed at a data review meeting by the principal investigator, statistician, project manager, medical staff and data management staff, and the IDMC will finalise the statistical analysis population.
Monitoring of adverse events
An AE is defined as any unfavourable experience that participants encounter during the study period, regardless of its relation to the treatment. Predefined AEs specific to the axilla include postoperative bleeding or infection, lymphoedema of the upper limb or chest wall, neuralgia, sensory abnormalities, decreased mobility, muscle weakness or pain in the arm and shoulder. The severity of AEs is categorised as mild, moderate or severe, based on the CTCAE V.5.0 criteria. An SAE is defined as an adverse medical occurrence or effect related to surgical treatment that results in hospitalisation, prolonged hospital stay or death.
In this study, the procedural risk is low, and the likelihood of an SAE is minimal. In the event of BCRL and radiotherapy-related adverse effects, symptomatic treatment is typically sufficient. The principal investigators of this study are responsible for evaluating SAE, which should be reported within 15 calendar days to the ethics committee. For fatal or life-threatening cases, there is a maximum of 7 days for an initial report and 8 days for a completed report. All SAEs should be followed up until they have subsided or reached a steady state. Follow-up may necessitate additional tests or medical procedures and/or referral to a general practitioner or medical specialist, depending on the specific event.
Ethics and dissemination
The study will be conducted in strict compliance with the principles of the Declaration of Helsinki, and data will be anonymised by removing identifiable information and using unique study codes. The study has received approval from the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (ethical review number: 2023-SR-169). Protocol amendments require prior ethics committee approval and trial registry notification from the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University, and these changes will be notified to the trial registry. The findings from the study will be disseminated through academic conferences and published in peer-reviewed journals.
Discussion
Although de-escalation procedures have replaced ALND as the standard surgical approach for the axilla in ypN0 patients, certain techniques such as TLNB still lack sufficient data on oncological outcomes, limiting their clinical application. To date, prognostic data13–17 on recurrence or survival outcomes are mainly available for patients who underwent SLNB or TAD, while safety data on TLNB requires further validation.
Ongoing trials and our SrLNB trial are expected to provide safety evidence for axillary de-escalation surgery and expedite its clinical application. The ATNEC (NCT04109079) study enrolls patients with cN1 and performs SLNB without ALND in apCR patients. The MINIMAX28 trial includes cN1-3 patients and axillary de-escalation surgery will be employed according to local protocol. The AXSANA29 study uses a variety of marking procedures with the goal of identifying the optimal procedure. Another ongoing trial, NSABP B51/RTOG 130430,30 evaluates whether adding RNI improves survival outcomes. These trials will provide further evidence on optimal locoregional treatment strategies for ypN0 disease regarding long-term prognosis, aiming to prevent both overtreatment and undertreatment. These trials will provide further evidence on optimal locoregional treatment strategies for ypN0 disease.
Compared with these studies, our SrLNB study offers several advantages: First, SrLNB has the benefits of using carbon tattooing, including eliminating the need for a second localisation prior to the surgery, avoiding the use of radioactive particles, and providing a long, identifiable period of black stained lymph nodes by carbon nanoparticles that lasts up to half a year. Secondly, our preliminary trial11 has confirmed a high detection rate and a FNR below the threshold value (10%). More importantly, our study places greater emphasis on axillary morbidity and QoL. BCRL rate and PRO will be reported using multiple objective and subjective measurements. This study also has limitations. It is a single-centre study with a limited sample size. We hope to expand the experimental centre and increase the number of enrolments after achieving preliminary results.
Trial status and time plan
The protocol version number and date are V.3.0 and 16 May 2023, respectively. The study protocol was developed in 2022. The first patient was enrolled on 11 September 2023, and the study is currently in the patient recruitment phase. Enrolment is projected to be completed by August 2025, with the 3-year follow-up expected to be preliminarily completed by October 2026.
Ethics statements
Patient consent for publication
Acknowledgments
We thank all participants who volunteered to take part in this study and all investigators who have contributed to this study.
References
Footnotes
LM, RC and MW contributed equally.
Contributors All authors are from The First Affiliated Hospital with Nanjing Medical University. JW and XZ were the principal investigators of this study, they proposed the preliminary conception and conduct a tentative design, financial support was also provided. LM and XL developed the detailed protocol, RC and MW helped to register the trial and obtain ethical approval. LW, RZ, JD, HY and XS were responsible for patient recruitment and data collection, YG, JD and YW were responsible for data checking and data monitoring. LM drafted the initial manuscript. JW is responsible for the overall content as guarantor. All authors critically revised the manuscript and approved the final study protocol.
Funding This research was partially supported by the Chinese Society of Clinical Oncology Foundation (Y-HR2020MS-0421), Jiangsu Province Elderly Health Research Project (LKM2022033, LKM2023023), China Breast Surgery Young Physician Research Award Advantage Support Programme (2020-CHPASLP-01).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.