Article Text
Abstract
Objectives To investigate long-acting injectable (LAI) antipsychotic prescribing patterns and their associations with transition and continuation of care and healthcare resource utilisation (HCRU) for patients with schizophrenia in the USA.
Design A retrospective cohort study.
Setting Electronic health record data from adults in the USA with schizophrenia were extracted from the NeuroBlu Database V.21R2.
Participants Adults (aged ≥18 years) with a schizophrenia diagnosis who initiated LAI antipsychotic treatment during psychiatric inpatient admission. The index date was the date of LAI initiation. Patients who had ≥1 primary, secondary or tertiary ICD-9/10 (International Classification of Diseases) diagnosis of schizophrenia at clinical sites that had both inpatient and outpatient facilities were included.
Primary outcome measures Transition-of-care (eg, risk of rehospitalisation, number of hospital readmissions, number of outpatient visits post discharge), continuation-of-care (eg, first treatment path after discharge, time to index LAI discontinuation and number of patients who restarted LAIs after discontinuation) and HCRU endpoints (eg, length of stay of index hospitalisation and estimated cost for psychiatric outpatient visits pre-index and post-index) were the primary outcome measures.
Results A total of 1197 patients were included who initiated an LAI in an inpatient setting. Of 339 patients with ≥3 months pre-index and post-index data, median time to rehospitalisation was 135 days. Patients discharged taking an LAI alone had lower frequency of rehospitalisation (incidence rate ratio (IRR)=0.62 (95% CI, 0.46 to 0.84)), lower risk of longer hospital stays (IRR=0.60 (95% CI, 0.43 to 0.84)), lower risk of becoming rehospitalised (HR=0.49 (95% CI, 0.35 to 0.69)) and lower risk of outpatient visits (IRR=0.50 (95% CI, 0.36 to 0.70)) versus patients co-prescribed an oral antipsychotic (LAI+OA). Patients discharged taking an LAI dosed once every 1–2 months or once every 2 weeks had lower frequency of rehospitalisation (IRR=0.85 (95% CI, 0.64 to 1.14)), lower risk of longer hospital stays (IRR=0.90 (95% CI, 0.70 to 1.15)) and lower risk of becoming rehospitalised versus an LAI dosed once every 2 weeks; risk of becoming rehospitalised was no different (HR=1.00 (95% CI, 0.76 to 1.32)) and risk of outpatient visits was greater (IRR=1.25 (95% CI, 0.96 to 1.63)). During hospitalisation, 73.4% of patients were co-prescribed an OA, most frequently risperidone, with their index LAI. From pre-admission to post-discharge, psychiatric clinic costs significantly increased (US$14 231, p<0.01 post-discharge vs pre-admission) among patients co-prescribed an OA. For patients who were prescribed an LAI alone there was minimal change in costs from pre-admission to post-discharge (p=0.068). At 12 months post-index, 75.3% of patients discontinued LAIs, dosed once every 1–2 months versus LAIs, dosed once every 2 weeks (86.5%) and median days to discontinuation was longer (67 (IQR 60–91) vs 32 (IQR 28–49).
Conclusions Patients prescribed a combination of LAI and OA at discharge had a higher risk of rehospitalisation compared with those prescribed LAI alone. Additionally, the study findings suggest that patients are more likely to be prescribed oral risperidone, the most frequently used second-generation OA, which may support an easier transition to an LAI of the same molecule.
- Adult psychiatry
- Schizophrenia & psychotic disorders
- Medication Adherence
- Medication Persistence
- HEALTH ECONOMICS
- Quality in healthcare
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Adult psychiatry
- Schizophrenia & psychotic disorders
- Medication Adherence
- Medication Persistence
- HEALTH ECONOMICS
- Quality in healthcare
STRENGTHS AND LIMITATIONS OF THIS STUDY
The cohort consisted of a large, real-world sample of inpatient admitted adults with a schizophrenia diagnosis across the USA.
This study is one of the few comprehensive real-world evidence analyses into long-acting injectable prescribing patterns and their associations with transition and continuation of care in the USA for inpatient admitted adults with schizophrenia.
Data on treatment paths after hospital discharge, as well as healthcare resource utilisation (HCRU) and other clinical outcomes, are presented here.
As the NeuroBlu Database is not linked to claims data, information about some variables (eg, economic, educational, insurance and non-psychiatric medication) is unavailable, which limits inferences that can be made about social determinants of health and the influence of concomitant medication on care, for example.
The HCRU assessment was limited to psychiatric specialty clinics only (with 75% of data from at least a decade ago), thereby reducing the generalisability of the results.
Introduction
Schizophrenia is a serious and enduring psychiatric disorder primarily treated with antipsychotics (APs). The majority of APs are administered as oral antipsychotics (OAs) taken daily. Although highly effective for the treatment of schizophrenia, a substantial proportion of patients discontinue OAs by 12 months,1 2 and it has been estimated that approximately two-thirds of patients have poor adherence to OAs.3
Considering that patients with schizophrenia struggle with treatment adherence overall, offering APs as long-acting injectable (LAI) formulations given once every 2 weeks to once every 6 months may result in improved adherence, reduced risk of relapse and better long-term outcomes.1 2 4 5 Recent large randomised controlled trials and observational studies have found that patients who initiate LAI treatment have 20–30% reductions in risk of rehospitalisation and relapse,6 7 emergency department visits8 and psychiatric hospitalisation,9 as well as a 30–40% reduction in all-cause mortality and morbidity.10 In addition, a recent meta-analysis demonstrated that compared with OAs, LAIs reduced relapse rates by 30% and discontinuation rates by 20–22%.4 Some literature has demonstrated no difference in discontinuation rates between OAs and LAIs, but these studies were in patients with early-phase schizophrenia,11 limiting the generalisability of the results to the majority of patients with schizophrenia, whose illness is more advanced.12
In addition, the American Psychiatric Association guidelines state that LAIs are an evidence-based and effective choice for patients who require maintenance treatment in order to prevent relapse and who have a history of poor or uncertain adherence.13 The guidelines also state that discussions about LAIs are appropriate when patients have experienced relapse while taking OAs, exhibit lack of insight regarding their need for treatment, have a comorbid substance abuse disorder or are transitioning between care settings (eg, inpatient discharge or release from a correctional facility).13 Expert consensus surveys have indicated that LAIs may also be appropriate earlier in the disease course as well as in younger patients.14 15
Despite LAIs being associated with better clinical outcomes and adherence compared with OAs, LAIs are underused in clinical practice, with some healthcare professionals believing that patients will not accept LAIs while also overestimating the adherence of their patients taking OAs.10 11 16 17 In addition, some physicians have less confidence with LAI use.11 18 Other issues that may limit LAI use include cost, availability of specific APs as an LAI formulation, challenges in switching from OAs and patient concerns about multiple injections.17 18 Complex initiation regimens (ie, loading doses and oral supplementation) and pill burden can also increase non-adherence.19 There are ongoing efforts to develop LAIs that address each of these issues, thereby facilitating their use in different care settings.20
Other factors such as type of facility (clinics vs tertiary hospitals), number of hospitalisations before LAI initiation, time from LAI prescription to initiation, mean standardised dosage of LAI, OA treatment duration and mean dosage before LAI initiation have also been shown to play a role in LAI discontinuation rate.21 There is also a lack of evidence on strategies for switching between LAIs and how oral supplementation should be applied, but the consensus has been that the new LAI should provide plasma concentrations that correspond to the previous LAI as much as possible.22 Oral tolerability with a new AP should be established first, and oral supplementation should only be given if an LAI does not reach therapeutic levels quickly. However, there are no clear guidelines on the time period needed to determine tolerability or best practices for oral supplementation.23 24
As mentioned, there is evidence that LAIs can reduce hospitalisation rates25–27; however, less is known about the impact of LAI administration frequency on transition and continuation of care in outpatient settings. Comparing dosing frequencies, LAIs administered once every 1–2 months have been shown to increase adherence and decrease the risk and incidence of discontinuation and hospitalisation compared with LAIs administered more frequently (ie, once every 2 weeks).21 28 For example, 42.5% of patients taking paliperidone as an LAI administered once every 1–6 months, 63.2% of patients taking haloperidol as a once-monthly LAI and 65.5% of patients taking risperidone as an LAI given once every 2–4 weeks discontinue treatment by 6 months.21 Longer-duration LAIs are also associated with lower risk of drug concentrations falling below therapeutic levels because of missed doses.29
A previous study that investigated LAI use during transition-of-care in patients with schizophrenia has demonstrated that being given LAIs is associated with better psychosocial functioning and less negative symptoms in patients, but they were more likely to be hospitalised compared with patients given OAs.30 In addition, being prescribed a first-generation (FG) LAI was associated with aggression, higher risk of adverse effects and increased hospitalisation compared with second-generation (SG) LAI treatment in patients.30 Other studies demonstrated that prescription rates of SG LAIs in community-based settings increased over time,31 and that only 30.6% of healthcare service providers prescribed to patients in an outpatient setting.32
Other studies investigating claims databases have also shown that in patients with early diagnosed schizophrenia, LAI use was very low (approximately 4%) and although initiation of LAIs was successfully completed, OAs were generally the first-line therapy.33 Factors that were predictive of LAI implementation included unsuccessful OA implementation and more monthly schizophrenia-related hospitalisation and emergency room visits.34 Healthcare resource utilisation (HCRU) and costs were considerably higher for patients who initiated LAIs later in their disease course, with primary costs being emergency department visits and other outpatient visits.35
This study was designed to generate real-world evidence into LAI prescribing patterns and their associations with transition and continuation of care in the USA for patients with schizophrenia by using electronic health records (EHRs). This study expands on previous work by investigating HCRU in relation to primary outcomes consisting of adherence and discontinuation rates for LAIs post hospital discharge and secondary outcomes consisting of LAI dosing frequency and characterising patterns of OA supplementation.
Methods
Study design and participants
This was a retrospective cohort study of adults aged ≥18 years with a schizophrenia diagnosis who initiated LAI treatment during a psychiatric inpatient admission/hospitalisation as recorded in the NeuroBlu Database V.21R2, a database of EHRs containing data from US mental healthcare providers operating an EHR called MindLinc with data collected between 1999 and 2020.36 Administrative claims databases often do not contain information regarding clinical assessments nor granular data about treatment changes occurring in different care settings; on the other hand, EHRs include data on a patient’s medical history and treatment outcomes,37 which can be used to understand the patient journey through different care settings and the long-term continuation of care in outpatient settings.
The study included patients who had ≥1 primary, secondary or tertiary ICD-9/10 (International Classification of Diseases) diagnosis of schizophrenia at clinical sites that had both inpatient and outpatient facilities (online supplemental table 1).
Supplemental material
The index date was defined as the date of first LAI administration during psychiatric hospitalisation. For transition-of-care and HCRU analyses, the pre-admission period was defined as up to 12 months before the inpatient admission where the first LAI was administered (index hospitalisation). The time between admission and LAI initiation was not included in the definition of the pre-admission period.
The post-discharge period was defined as up to 12 months after discharge from the hospital. Patients with ≥3 months of pre-admission and post-discharge data were included in the transition-of-care analysis. For continuation-of-care analyses, the post-index period was defined as 12 months after index LAI initiation. Patients with ≥12 months of post-index data were included in the continuation-of-care analysis (figure 1).
Study design. *Among patients with ≥3 months of pre-admission and post-discharge data. LAI, long-acting injectable antipsychotic.
Variables and measurements
Transition-of-care endpoints included risk of rehospitalisation (hazard ratio [HR]), number of hospital readmissions (incidence rate ratio (IRR)) and number of outpatient visits (IRR) post discharge. In addition, HCRU endpoints included duration of hospitalisation (IRR) and estimated cost for psychiatric outpatient visits pre-index and post-index. Estimated costs were calculated using data derived from the Healthcare Cost and Utilization Project (HCUP),38 the largest collection of longitudinal hospital care data in the USA. The HCUP provides the total number of discharges, mean and median length of stay and median healthcare costs per year by individual ICD-9 codes for the national inpatient sample of 2014. Costs were adjusted for inflation per the Consumer Price Index39 to estimate median per-day hospitalisation costs and per-visit outpatient costs for schizophrenia in 2019 (US$3095 and US$126, respectively).
Continuation-of-care endpoints included first treatment path after discharge, time to index LAI discontinuation and number of patients who restarted LAIs after discontinuation (with an allowable gap of 2× the dosing interval from last injection date) or supplemented their LAI with an OA. Figure 2 indicates the decision tree to determine the first treatment path after discharge; patients could continue the index LAI, change to another LAI, change to an OA or discontinue the index LAI.
Possible treatment paths after discharge. LAI, long-acting injectable antipsychotic; OA, oral antipsychotic.
Statistical analysis
To compare independent groups, χ2 or Fisher’s exact tests were used for categorical variables and the Welch unpooled variance t-test or Mann-Whitney U test were used for continuous variables. For paired groups, the McNemar test was used for categorical variables. Continuous and count variables were tested for normality; the means were compared using paired t-tests for normally distributed data, and the medians were compared using the Wilcoxon signed-rank test for skewed data. All statistical tests were conducted at a significance level of p=0.05. The Clinical Global Impression-Severity (CGI-S) scale was used to determine illness severity (stratified as 1–3 (normal to mildly ill), 4–5 (moderately to markedly ill) and 6–7 (severely to most ill)) at admission and discharge. The CGI-S scale has been shown to be a reliable and valid clinical outcome appropriate for routine use in inpatient settings.40 To compare outcomes between patients who initiated LAIs with different treatment frequencies, Cox regression was used to model time to rehospitalisation with relevant baseline demographic, clinical, pre-index HCRU and treatment variables as potential confounders selected based on clinical relevance. These variables were further filtered based on statistical significance using stepwise regression. Generalised linear models were used to model HCRU variables. A negative binomial model was used, and residuals were analysed, to check for the fulfilment of model assumptions. To account for variations in follow-up time, an offset term was used to model the rates and the rounded-up, annualised number of rehospitalisations were reported for patients who did not have complete data availability for the whole 12-month post-discharge period. To account for missing covariate data, complete case analysis was used; however, for both race and CGI-S, missing data were categorised as unknown.
Patient and public involvement
There was no patient or public involvement during the design, conduct or reporting of the study.
Results
Patient selection and characteristics
Among 538 565 patients included in the NeuroBlu Database, only 2450 patients with schizophrenia diagnosis were prescribed an LAI and 1197 met the study inclusion criteria; 339 patients had ≥3 months of pre-admission and post-discharge data and were included in the transition-of-care and HCRU analyses, 449 patients had ≥12 months of post-index data and were included in continuation-of-care analyses (table 1). The mean age of the population was 40.2 years, with the majority being black (46.6%), men (66.3%) and from the Northeast USA (63.5%) (table 2). The most common dosage at initiation for each LAI was as follows: most patients were initiated on 25 mg risperidone (once every 2 weeks) (49%), 234 mg paliperidone once monthly (59%), 400 mg aripiprazole once monthly (81%), 100 mg haloperidol once monthly (50%) and 25 mg fluphenazine (every 4–6 weeks) (58%). In addition, initiation requirements for risperidone (oral supplementation) and paliperidone (initiation with loading dose of 234 mg and then switching to lower dose) were only met in 55% of patients prescribed risperidone and in 38% patients prescribed paliperidone.
Selection of study population
Patient demographics and clinical characteristics
Patient characteristics at admission
At admission, 157 patients were already taking an SG OA (36.0% risperidone). As it was not a requirement for patients to have pre-admission data, only 658 (55%) patients had visits recorded before the index hospitalisation, of whom 70.0% had a pre-admission history of psychiatric hospitalisation. Of the 1197 total patients, 887 (74%) patients had CGI-S data (mean score (SD) of 5 (1.0)), of whom 33.7% were severely ill (CGI-S score 6–7), 61.0% moderately ill (CGI-S score 4–5) and 5.3% mildly ill (CGI-S score 1–3) (table 2). Substance use disorder (31.2%), schizoaffective disorder (27.9%) and bipolar disorder (12.5%) were the most prevalent psychiatric comorbidities among the population (table 2).
Treatment patterns during hospitalisation and at discharge
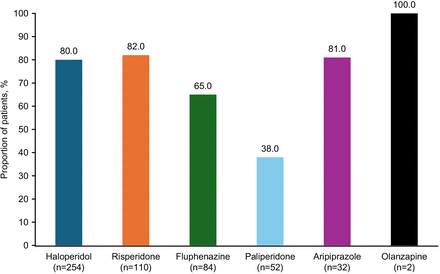
On average, LAIs were initiated 7 days post admission. The most prescribed index LAIs were haloperidol, dosed once monthly (49.2%; median dose strength, 100 mg/day) and risperidone, dosed once every 2 weeks (18.2%; median dose strength 25 mg/day), followed by fluphenazine, dosed once every 4–6 weeks (17.3%; median dose strength 25 mg/day), paliperidone palmitate (10.4%; median dose strength 234 mg/day) and aripiprazole (4.3%; median dose strength 400 mg/day), both dosed once monthly, olanzapine, dosed once every 2–4 weeks (0.4%) and aripiprazole lauroxil, dosed once every 2 months (0.1%); median dose strengths were not calculated for olanzapine and aripiprazole lauroxil because of the small sample size (figure 3). Even though there was a shift toward use of SG APs across time periods (overall 9% in 1999–2004 and 47% in 2015–2020), FG AP (ie, haloperidol and fluphenazine) use has remained high (91% in 1999–2004 and 53% in 2015–2020) (table 2). During hospitalisation, 73.4% of patients were co-prescribed an OA with their index LAI, and at discharge, 44.6% of patients were co-prescribed an OA with their index LAI (table 2); the co-prescribed OAs were the same dosages. Between admission and LAI initiation, 538 patients were prescribed SG OAs (50.0% risperidone). Most of the patients who were co-prescribed an OA at discharge were co-prescribed the same OA molecule (65–100%) as their index LAI, except for paliperidone (38%) (figure 4).
Treatment patterns pre-admission and during hospitalisation. *Other LAIs included olanzapine q2w–q4w and aripiprazole lauroxil q2m. LAI, long-acting injectable antipsychotic; OA, oral antipsychotic; q1m, once monthly; q2m, once every 2 months; q2w, once every 2 weeks; q4w, once every 4 weeks; q6w, once every 6 weeks.
Patients co-prescribed the same molecule as OA and LAI at discharge (for patients who were co-prescribed OA+LAI). LAI, long-acting injectable antipsychotic; OA, oral antipsychotic.
Healthcare resource utilisation
Among patients co-prescribed an OA with their index LAI at discharge, 74.1% were rehospitalised within 12 months versus 68.5% of patients prescribed their index LAI alone (figure 5). Post discharge, patients prescribed their index LAI as monotherapy (n=156) had a 51% lower risk of becoming rehospitalised (adjusted HR=0.49 (95% CI, 0.35 to 0.69), p<0.01), 38% lower frequency of rehospitalisation (adjusted IRR=0.62 (95% CI, 0.46 to 0.84)) and lower risk of outpatient visits (adjusted IRR=0.50 (95% CI, 0.36 to 0.70)) than those co-prescribed an OA (n=183). The median number of outpatient visits increased from pre-admission to post-discharge among patients discharged with an LAI dosed once every 1–2 months (haloperidol, fluphenazine, paliperidone palmitate, aripiprazole and aripiprazole lauroxil) from 0 (IQR, 0–13) to 1 (0–32; p<0.01), whereas there was no increase among patients discharged with an LAI dosed once every 2 weeks (risperidone; 0 (0–7.75) to 0 (0–8); p=0.08); however, the difference between post-discharge values was not significant (p=0.10). Patients discharged taking an LAI dosed once every 1–2 months or once every 2 weeks had lower frequency of rehospitalisation (IRR=0.85 (95% CI, 0.64 to 1.14)), lower risk of longer hospital stays (IRR=0.90 (95% CI, 0.70 to 1.15)) and lower risk of becoming rehospitalised versus an LAI dosed more often; risk of becoming rehospitalised was no different (HR=1.00 (95% CI, 0.76 to 1.32)) and risk of outpatient visits was greater (IRR=1.25 (95% CI, 0.96 to 1.63)). From pre-admission to post-discharge, the median (IQR) number of outpatient visits significantly increased from 4 (0–23.5) to 8 (0–45; p<0.01) and psychiatric clinic costs significantly increased from US$14 490 (US$3095–US$52 615) to US$28 721 (US$6245–US$81 147; difference of US$14 231, p<0.01) among patients co-prescribed an OA at discharge, whereas there was minimal change among patients discharged with an LAI alone (0 (0–0) to 0 (0–2), p=0.08 and US$6190 (US$0–US$23 573) to US$6190 (US$95–US$35 710); no difference, p=0.68).
Rehospitalisation rate within 12 months. HR in the rehospitalisation model was adjusted for pre-index HCRU, region and index hospitalisation length of stay. HCRU, healthcare resource utilisation; LAI, long-acting injectable antipsychotic; OA, oral antipsychotic.
Continuation of care
At 12 months post-index, a smaller proportion of patients (75.3%) discontinued index LAIs dosed once every 1–2 months (haloperidol, fluphenazine, paliperidone palmitate, aripiprazole and aripiprazole lauroxil) compared with index LAIs dosed once every 2 weeks (86.5%; risperidone) (figure 6). The median time to discontinuation of index LAI was 67 days (IQR, 60–91) for LAIs dosed once every 1–2 months and 32 days (28–49) for those dosed once every 2 weeks.
Comparison of first treatment path (LAI continuation compared to treatment discontinuation) post discharge by LAI dosing interval. LAI, long-acting injectable antipsychotic.
There were no statistically significant differences (p>0.05) in mean (SD) CGI-S scores between patients prescribed their index LAI alone at discharge (4.5 (1.1)) and patients co-prescribed an OA at discharge (4.6 (0.9)). However, a larger proportion of patients who had been co-prescribed an OA (46%) versus an LAI alone (27%) showed improvements in CGI-S from admission to discharge. This could be a reflection of differences in disease/relapse severity, with the former including a larger proportion of patients (40%) who were severely ill at admission compared with those prescribed an LAI alone at index (29%).
Discussion
This study used real-world data to investigate the use of LAIs during hospital admission and the transition and continuation of care after discharge. HCRU patterns associated with LAI prescriptions were also assessed. The most prescribed index LAIs were haloperidol, dosed once monthly and risperidone, dosed once every 2 weeks. During hospitalisation, nearly three-quarters of patients were co-prescribed an OA at LAI initiation, while at discharge, about half of patients were co-prescribed an OA. Even though the reason for OA co-prescription (ie, temporary supplementation vs ongoing co-prescription) was not documented in the database, based on the dosages of the co-prescribed OAs and the high proportion of patients prescribed the same OA molecule as the LAI, it is likely that the co-prescribed OAs were used during LAI initiation as a supplement to attain therapeutic levels more quickly. Expert consensus surveys have indicated that the time required for oral supplementation should be guided by the duration needed for attainment of therapeutic LAI levels.23 However, outside of this LAI initiation period, there is limited evidence that co-prescription of an OA with an LAI has increased benefit compared with LAI monotherapy and the combination of OA and LAI could lead to increased side effect burden and subsequent issues with poor adherence and risk for relapse.1 41 The reported prevalence of AP co-prescription in hospitalised patients ranges from 20% to 66%, and this practice appears to occur primarily with patients with schizophrenia.42–44 A recent systematic review highlighted the prevalence of AP polypharmacy in mental health disorders (of which 52% were schizophrenia spectrum disorders), which has increased significantly from 1970 to 2023 and was higher among inpatients than outpatients (31% vs 20%, respectively). The review also showed that AP polypharmacy was associated with increased risk of relapse, hospitalisation, worse global functioning and higher risk of adverse events compared with AP monotherapy.45
The median time to LAI initiation during hospitalisation was 7 days. This lag may reflect the time needed for patients to be stabilised on OAs before being transitioned to LAIs, and to establish tolerability. This allows clinicians to observe patients and assess whether patients are responsive to and tolerant of the AP; however, there may be an unmet need for LAIs that can reach therapeutic plasma concentrations more rapidly without a complex initiation regimen (which can help decrease the burden on HCRU and related healthcare costs), as evidenced by a previous meta-analysis.19
From pre-admission to post-discharge, the median increase in outpatient visits and psychiatric clinic costs were significantly higher among patients co-prescribed an OA compared with patients prescribed their index LAI alone at discharge. Ongoing OA and LAI co-prescription could be related to a higher illness severity or complexity, which could plausibly cause increased HCRU. Co-prescription of OAs and LAIs could also lead to a higher burden of side effects, which may require more outpatient visits to adjust medication doses46 or manage side effects.
Overall, haloperidol was the most common LAI initiated during hospitalisation. This FG AP has been used for several decades, whereas newer SG LAIs have only been in use for the last 1–2 decades. Since this study included data from 1999 onwards, FG LAIs might be overrepresented in this study. Despite the increased use of SG LAIs in more recent time periods (overall 9% in 1999–2004 and 47% in 2015–2020), haloperidol and fluphenazine LAI usage remained high (91% in 1999–2004 and 53% in 2015–2020). This was also shown by other US studies that have found that haloperidol and fluphenazine continue to be commonly used (perhaps because of their low cost, accessibility and beneficial effects on aggression in schizophrenia47 48), despite being associated with side effects such as extrapyramidal symptoms that could affect adherence.49 In contrast, SG LAIs are better tolerated with improved side-effect profiles,50 increased treatment adherence51 52 and lower discontinuation rates53; therefore, patients should be educated and given the choice of SG LAIs where appropriate.
Regarding treatment discontinuation, 75.1% of patients discharged with LAIs dosed once every 1–2 months (median time to discontinuation, 67 days) discontinued their index LAI treatment compared with LAIs dosed once every 2 weeks (87%; 32 days, p<0.01). The median time to index LAI discontinuation was longer in patients discharged with an index LAI dosed once every 1–2 months compared with an index LAI dosed once every 2 weeks, likely related to reduced adherence demands inherent to longer administration intervals. This supports previous literature that reported increased treatment adherence and persistence with LAIs dosed once monthly versus more often.54
In terms of HCRU, there was a significant difference (p=0.01) in the number of outpatient visits from pre-admission (median of 0) to post-discharge (median of 1) with an LAI dosed once every 1–2 months; however, differences in rehospitalisation rates between dosing frequency groups were not significant, despite poorer LAI adherence rates in patients taking an LAI dosed once every 2 weeks. This could be because adherence differences between groups were not sufficiently large enough to be statistically significant. For patients co-prescribed an OA, HCRU and associated costs were higher, compared with patients who were prescribed their index LAI alone. This could be related to factors other than adherence (ie, patients discharged with an LAI co-prescribed with an OA may have had more severe disease and/or comorbid psychiatric illness, even though these variables were controlled for in the analysis).
The NeuroBlu Database does have some limitations that reduce the generalisability of the results. Specifically, NeuroBlu does not contain medical records from primary care settings, and thus HCRU was only assessed in psychiatric specialty clinics, including community mental health clinics. Also, the database is also not linked to claims data; therefore, it can only be assumed that patients filled their own prescriptions. Other variables, such as economic, educational, insurance and non-psychiatric medication information were also not available in the database and therefore could not be included as confounders in the regression analysis. In addition, due to the descriptive nature of this work and limitations of the real-world data set, there was no adjustment for confounders among certain observed relationships. For example, we did not control for the confounding influence of illness severity when comparing outcomes between patients who were prescribed LAIs versus OA+LAI, or for the influence of dosing frequency and strength on CGI-S improvement in the context of LAI co-prescription. Also, about 75% of the data in NeuroBlu are from at least a decade ago, and the LAI landscape has changed substantially since that time. Other limitations of the study are that the classification of the treatment paths was estimated at a population level based on external expert inputs, and therefore, it is difficult to determine the treating clinician’s true intention with their prescribing decisions for a specific patient. Additionally, patients would not be considered to have discontinued LAI if they received doses within the dosing window, regardless of rehospitalisation. This could potentially underestimate the proportion of patients who discontinued treatment and influence the differences in discontinuations between the LAI+OA and LAI-alone group. Also, the LAI dosing profile groupings (ie, once every 1–2 months vs once every 2 weeks) were determined at the product level. In the case of the olanzapine LAI, which can be dosed once every 2 weeks or once monthly, this resulted in three patients who received olanzapine once monthly and one patient with missing dosing information being potentially misclassified and included in the once-every-2-weeks group. Given the small number of patients affected, this is not expected to substantially influence results. Another consideration is that common schizophrenia comorbidities may be under-represented in this sample. The prevalence of post-traumatic stress disorder (PTSD; 2%), major depressive disorder (MDD; 6%) and obsessive-compulsive disorder (OCD; 1%) in this hospitalised population was much lower than in the general mental health community and other inpatient samples, where average reported prevalence was 29% for PTSD, 50% for MDD and 23% for OCD.55 This likely reflects differences in disease severity between patients in the community and other inpatient settings, and limits the generalisability of these results to routine clinical care/maintenance treatment. Lastly, although the study period was ≤12 months before admission to ≤12 months after discharge, patients with as little as 3 months of data were included in the transition-of-care analyses to maintain sufficient sample size, which introduces the risk of bias from most patients having limited follow-up data, even though it has been statistically adjusted for in the analyses.
Conclusions
This real-world evidence study demonstrated that patients prescribed a combination of LAI and OA at discharge had a higher risk of rehospitalisation compared with those prescribed LAI alone. In addition, HCRU and associated costs were higher for patients who were co-prescribed an OA with their index LAI. This warrants further exploration to distinguish illness severity from other causes of rehospitalisation (eg, increased side effect burden which could negatively impact treatment adherence and subsequently increase risk of relapse). Increased risk of rehospitalisation and treatment discontinuation could also be due to a combination of underlying illness severity and subsequent risk of relapse together with increased risk of adverse events (due to higher antipsychotic plasma concentrations). LAIs with less frequent administration, particularly those administered once monthly or less frequently, were associated with reduced discontinuation rates. Another finding was that risperidone was the most prescribed SG OA and LAI, which demonstrated that molecules that have an LAI formulation may make it easier when transitioning patients. SG LAIs are also better tolerated with increased treatment adherence, and lower discontinuation rates, compared with FG LAIs such as haloperidol. Use of LAIs that support earlier initiation during hospitalisation should also be considered when developing a treatment plan. Future research should focus on longer-term outcomes with more comprehensive modelling of confounders or mediating factors.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
NeuroBlu Database data have been de-identified and normalised in accordance with Observational Health Data Sciences and Informatics data standards. The NeuroBlu Database platform has received a waiver of authorisation to use de-identified healthcare data from the WCG (WIRB-Copernicus Group) Institutional Review Board (Ref: WCG-IRB 1-1470336-1). Therefore, informed consent was not required for the purposes of this study.
Acknowledgments
This study was supported by funding from Teva Branded Pharmaceutical Products R&D LLC. Medical writing and editorial support were provided by Jean-Paul Fouche, PhD, Jennifer C Jaworski, MS, BCMAS, CMPP, and Kelsey Gribbon, MS, all of Ashfield MedComms, an Inizio company, and were funded by Teva Branded Pharmaceutical Products R&D LLC.
References
Footnotes
X @RPatelDr
Contributors RP, CL, MO, SK, AJ, ST, RTH, MJP and KRF contributed to the conceptualisation of the manuscript. RP contributed to supervision. CL, MO, SK and AJ contributed to data curation. KRF contributed to data interpretation. CL, MO, SK and AJ contributed to formal analysis, investigation and methodology. All authors contributed to writing—review and editing. ST is guarantor.
Funding This work was funded by Teva Branded Pharmaceutical Products R&D LLC.
Competing interests This study was supported by funding from Teva Branded Pharmaceutical Products R&D LLC. CL, SK, MO and AJ are employee(s) of Holmusk Technologies at the time of the study, which has received payments from Teva Pharmaceuticals in relation to this study. ST, KRF, RTH and MJP are employees and/or shareholders of Teva Pharmaceuticals. RP has received grant funding from the National Institute for Health and Care Research (NIHR301690), the Medical Research Council (MR/S003118/1), the Academy of Medical Sciences (SGL015/1020) and Janssen, and consulting fees from Holmusk, Akrivia Health, Columbia Data Analytics, Clinilabs, Boehringer Ingelheim, Teva and Otsuka.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.