Article Text
Abstract
Objectives Vitamin D deficiency is prevalent among the population. Previous studies have shown that vitamin D supplementation might be useful for treating COVID-19 infection. Therefore, we performed a meta-analysis to explore vitamin D supplementation efficacy in treating COVID-19 patients with vitamin D deficiency.
Design Systematic review and meta-analysis
Data sources PubMed, Cochrane Library, Embase and Web of Science.
Eligibility criteria Randomised controlled trials exploring vitamin D supplementation for patients with COVID-19 and vitamin D deficiency.
Data extraction and synthesis Two independent reviewers employed standardised methods to search, screen and code the included studies. The primary outcomes included mortality during follow-up, 28-day mortality, need for mechanical ventilation and intensive care unit (ICU). The secondary outcome included length of stay in hospital and ICU. The risk of bias was assessed using the Risk of Bias 2 tool. Depending on the level of heterogeneity, either a random-effects model or a fixed-effects model was applied. The findings were summarised using Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profiles and synthesised qualitatively.
Results A total of nine studies, comprising 870 participants, were included in the analysis. The pooled results indicated that vitamin D supplementation was associated with a lower risk of mortality (risk ratio 0.76; 95% CI 0.60 to 0.97). However, this apparent benefit was not robust when examined through the leave-one-out method and trial sequential analysis. Regarding other outcomes, there was no statistically significant difference between vitamin D supplementation and no supplementation in terms of 28-day mortality, the need for mechanical ventilation and ICU admission. Vitamin D supplementation was associated with a 0.41 day shorter length of stay in the ICU (mean difference −0.41; 95% CI −1.09 to 0.28) and a 0.07 day shorter length of stay in the hospital (mean difference −0.07; 95% CI −0.61 to 0.46) compared with no supplementation; however, neither difference was statistically significant.
Conclusion Based on evidence of low to moderate quality, vitamin D supplementation reduced the mortality rate during follow-up in COVID-19 patients with vitamin D deficiency. However, it did not improve 28-day mortality, nor did it reduce the need for mechanical ventilation and ICU admission, or the length of stay in the ICU and hospital.
PROSPERO registration number CRD42024573791.
- COVID-19
- Meta-Analysis
- NUTRITION & DIETETICS
- Health
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This meta-analysis of randomised controlled trials (RCTs) was conducted and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses checklist.
A comprehensive literature search was performed across multiple databases to identify relevant studies.
Rigorous inclusion criteria were applied to ensure the quality and relevance of studies.
Trial sequential analysis and sensitivity analysis were used to assess the statistical robustness of the results.
The number of studies included was limited, with only nine RCTs and relatively small sample sizes, which may affect the generalisability of the results.
Introduction
COVID-19, caused by the SARS-CoV-2 virus, is a highly transmissible and potentially severe respiratory illness that has resulted in a global pandemic, affecting millions of people worldwide with varying morbidity and mortality rates.1 2
Vitamin D, a steroid hormone derived from cholesterol, plays a significant role in regulating the expression of various genes, including those in immune cells.3 In hospitalised COVID-19 patients, vitamin D also showed anti-inflammatory effects.4 Vitamin D deficiency is widespread across the globe; for example, 40% of the European population is reported to lack sufficient vitamin D, and vitamin D deficiency is also common in high-altitude regions such as Nepal, the Andes and Tibet.5 6 Maintaining appropriate levels of vitamin D is essential for optimal respiratory immune function.3 7–11 Despite this, the precise impact of vitamin D supplementation on preventing and treating COVID-19 remains a topic of debate. According to a systematic review, vitamin D supplementation can significantly reduce the severity of COVID-19 infection, as measured by outcomes such as hospitalisation rates, the need for mechanical ventilation and mortality, suggesting its use as a supplementary treatment for COVID-19.12 In contrast, a 2021 meta-analysis that included eight randomised controlled trials (RCTs) found that vitamin D supplementation did not enhance clinical outcomes in patients infected with SARS-CoV-2.13 Recently, a meta-analysis conducted by Meng et al explored the role of vitamin D in the prevention and treatment of SARS-CoV-2 infection. Their results suggested that vitamin D supplementation may have some beneficial impact on the severity of illness caused by SARS-CoV-2, particularly in vitamin D-deficient patients. Although they specifically analysed patients with vitamin D deficiency, the studies they included were limited, and the analysis focused solely on mortality as the outcome. Moreover, they did not perform comprehensive subgroup analyses, such as based on the severity of vitamin D deficiency.
Amrein et al raised another important point, namely that vitamin D is clearly not a cure-all and is likely effective only when there is a deficiency.6 To comprehensively investigate the role of vitamin D supplementation in these patients, we conducted a meta-analysis of RCTs to determine whether vitamin D supplementation improves clinical outcomes (mortality during follow-up, 28-day mortality, need for mechanical ventilation and ICU and length of stay in hospital and ICU) in COVID-19 patients with vitamin D deficiency.
Methods
This meta-analysis of RCTs was reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis checklist.14
Search strategy and selection criteria
A comprehensive literature search was conducted on 1 June 2024 across several databases including PubMed, Cochrane Library, Embase and Web of Science with MeSH terms and broad search terms. We also manually searched the reference lists of relevant review articles. After completing the initial research, we conducted the same search again to include the latest published studies. The detailed search strategy was provided in the appendix. The retrieved literature was imported into EndNote X9. After removing duplicate references, it was assessed for eligibility by two reviewers. Based on the PICO principle, the inclusion criteria we applied are as follows:
P: COVID-19 patients with vitamin D deficiency;
I: standard care plus vitamin D supplementation;
C: standard care;
O: mortality rate, need for mechanical ventilation or ICU admission, length of stay in ICU and hospital.
Exclusion criteria were as follows: non-RCTs and studies for which full text could not be retrieved. The definition of vitamin D deficiency was according to previous studies.6 15–17 Any disputes will be resolved through discussion.
Data extraction
A comprehensive data extraction form was developed based on the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions. The form was piloted on a subset of the included studies before extracting the following data: author details, participant characteristics, intervention details (type, duration, frequency and other details), primary and secondary outcomes and follow-up times.
The consistency between data extractors was measured using the Kappa value. Any disputes will be resolved through discussion.
Quality assessment
Potential sources of bias in RCTs were assessed using Risk of Bias 2 (Rob2), a revised tool for assessing the risk of bias in randomised trials.18 Rob2 encompasses five key domains: (1) randomisation process; (2) deviations from intended interventions; (3) missing outcome data; (4) measurement of the outcome and (5) selection of the reported result. Within each domain, bias was evaluated and categorised as either low risk, some concerns or high risk, depending on the circumstances and relevant evidence. Ultimately, the overall bias of each study was classified as either low risk, some concerns or high risk, based on the comprehensive assessment of bias across the five domains. When there was a discrepancy in the assessment results for a certain domain, the outcome was resolved through discussion.
Outcomes
The primary outcomes were mortality during follow-up and 28-day mortality. The secondary outcomes included the need for mechanical ventilation and ICU admission, length of stay in hospital and ICU. Mortality during follow-up refers to the deaths that occurred during the follow-up period in each study. Since the follow-up durations vary across studies, the time frame for mortality during follow-up is not consistent; 28-day mortality specifically refers to the mortality rate from the start of the study up to day 28. The need for mechanical ventilation and ICU admission refers to patients who initially did not require mechanical ventilation or ICU admission but received mechanical ventilation or were admitted to the ICU during the study. Length of stay in hospital and ICU refers to the duration of hospitalisation and ICU stay for patients who received different treatments.
Statistical analysis
Dichotomous variables were presented as event numbers and total numbers, with combined outcomes expressed as risk ratio (RR) with 95% CIs. Continuous variables were presented as mean and SD, with combined outcomes expressed as mean difference (MD) with 95% CIs. The choice of analysis model was based on the level of heterogeneity. If I² ≥ 50%, heterogeneity was considered significant, and the DerSimonian-Laird method combined with a random-effects model was used for analysis. If I² < 50%, no significant heterogeneity was assumed, and the inverse-variance method combined with a fixed-effects model was used for analysis.19 Subgroup analysis according to different characteristics (severity of COVID-19, vitamin D supplementation, definition of vitamin D deficiency and so on) was conducted on mortality during follow-up. Sensitivity analysis was performed using the leave-one-out method. A funnel plot was generated to subjectively assess publication bias, and Egger’s test was also conducted to objectively test for publication bias; if p>0.05, no significant publication bias was assumed. In this study, trial sequential analysis was performed using Trial Sequential Analysis software (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet) (http://ctu.dk/tsa/). The meta-analysis was performed using Stata V.17 (STATA Corporation, Texas, USA) (https://www.stata.com/stata17/). The quality of evidence was assessed by Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines.20
Patient and public involvement
None.
Results
Literature search
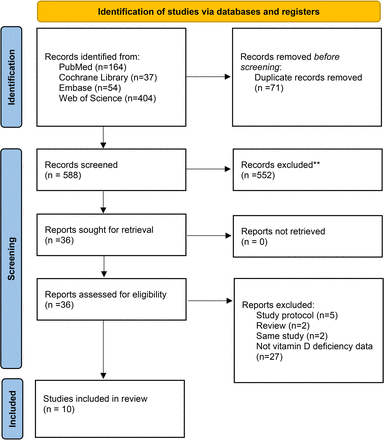
A total of 659 studies were initially found across all databases, with 71 identified as duplicates. After screening titles and abstracts, 552 studies were excluded. The remaining 36 studies were then assessed for full text. Ultimately, 10 studies15–17 21–27 met the inclusion criteria and were included in the analysis (figure 1).
Flowchart of literature search.
Baseline study characteristics
A total of 10 studies,15–17 21–26 encompassing 870 participants, were included. The vitamin D dosage ranged from 3000 IU to 200 000 IU. Three studies used a single high dose of vitamin D supplementation, while seven studies employed a continuous dosing regimen. Seven studies defined vitamin D deficiency as <20 ng/mL, two studies as <30 ng/mL and one study as <10 ng/mL. Additionally, two studies focused on severe COVID-19, and two studies examined moderate to severe COVID-19 cases (table 1).
Characteristic of included randomised controlled trials
Quality assessment
We evaluated the outcomes reported in the studies. We found that among the 28 relevant outcomes, 14 were classified as low risk and 14 as having some concerns. For example, the study by Soliman et al did not provide detailed information on the randomisation method, which raised concerns about the randomisation process. In the studies by Singh et al and others, vitamin D deficiency was defined as <10 ng/mL, while Cervero et al and Maghbooli et al defined deficiency as <30 ng/mL, which differed from the commonly accepted definition of deficiency. Therefore, these studies also carried an overall risk of bias. The detailed distribution of bias is shown in online supplemental eTable 1.
Supplemental material
The Kappa value, used to estimate the equivalence of data extraction in this study, was 0.86.
Mortality
Nine studies reported the mortality during follow-up. The pooled result showed that the risk of death in the vitamin D group was 24% lower than in the non-supplementation group (RR 0.76; 95% CI 0.60 to 0.97) (figure 2).
Vitamin D supplementation vs no vitamin D supplementation on mortality during follow-up, 28-day mortality, need for mechanical ventilation and need for ICU admission. ICU, intensive care unit.
To assess the role of vitamin D in reducing hospitalisation mortality, we analysed 28-day mortality. The pooled result showed that the risk of mortality was 21% lower in the vitamin D group, but this difference was not statistically significant (RR 0.78; 95% CI 0.55 to 1.38) (figure 2).
Need for ICU admission and mechanical ventilation
Three studies reported on the need for mechanical ventilation, and the pooled results showed that the need for mechanical ventilation was 10% lower in the vitamin D group, but this difference was not statistically significant (RR 0.90; 95% CI 0.69 to 1.17) (figure 2).
Four studies reported on the need for ICU admission, and the pooled results showed the need for requiring ICU care was 12% lower in the vitamin D group, but this difference was not statistically significant (RR 0.88; 95% CI 0.51 to 1.52) (figure 2).
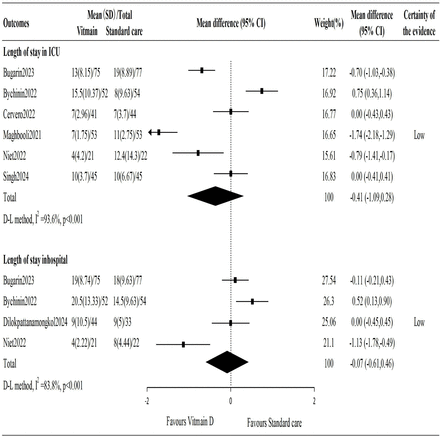
Length of stay in ICU and hospital
Six studies reported on the length of stay in the ICU, and the pooled results showed that the average length of ICU stay was 0.41 days shorter in the vitamin D group, but this difference was not statistically significant (MD −0.41 days; 95% CI −1.09 to 0.28).
Four studies reported on the length of stay in the hospital, and the pooled results showed the average hospital stay was 0.07 days shorter in the vitamin D group, but this difference was also not statistically significant (MD −0.07 days; 95% CI −0.61 to 0.46) (figure 3).
Vitamin D supplementation vs no vitamin D supplementation on length of stay in ICU and hospital. ICU, intensive care unit.
Subgroup analysis
Considering the limited number of included studies, we performed a subgroup analysis only on mortality during follow-up. Considering that participants’ responses to vitamin D may vary due to differences in the severity of COVID-19, supplementation frequency, degree of vitamin D deficiency, development level of the country, risk of bias and sample size across studies, we performed subgroup analyses based on these characteristics (figure 4). There were no statistically significant group differences within any of the subgroups, so these results do not support an effect of the aforementioned characteristics on vitamin D.
Subgroup analysis of mortality during follow-up.
Sensitivity analysis
Sensitivity analysis was performed on mortality during follow-up by leave-one-out method and trial sequential analysis.
Sensitivity analysis was performed on mortality during follow-up using the leave-one-out method and trial sequential analysis (online supplemental eFigure 1).
Using the leave-one-out method, we found that excluding the studies by Burgarin et al, Bychinin et al,21 Maghbooli et al15 and Singh et al17 resulted in no statistically significant difference between vitamin D supplementation and no vitamin D supplementation. This suggests that the result was not robust.
We also performed a trial sequential analysis on mortality during follow-up. With 80% power, the pooled result showed no statistically significant difference (RR 0.74; α-spending adjusted CI 0.46 to 1.19). The required sample size was determined to be 1874 (online supplemental eFigure 2).
Publication bias
We plotted funnel plots for the aforementioned outcomes (online supplemental eFigure 3–8). However, due to the limited number of included studies, there is a considerable risk of bias when evaluating the symmetry of the funnel plots. To more objectively assess publication bias, we also performed Egger’s test. The p-values for Egger’s test for the above outcomes were all greater than 0.05, indicating no significant evidence of publication bias.
Grade assessment
The quality of evidence for the above outcomes ranged from very low to moderate (online supplemental eTable 2). Specifically, the quality of evidence was moderate for mortality during follow-up, 28-day mortality, need for mechanical ventilation and need for ICU admission. In contrast, the quality of evidence was low for length of stay in ICU and length of stay in hospital.
Discussion
Our study comprehensively explored the efficacy of vitamin D in treating COVID-19 patients with vitamin D deficiency. We found that vitamin D supplementation could reduce mortality during follow-up. However, this result should be interpreted with caution for the following reasons. First, the leave-one-out method showed that nearly half of the studies could change the conclusion, indicating that the result was not robust. Second, in the subgroup analysis, most groups showed no statistically significant difference between vitamin D supplementation and no vitamin D supplementation. This may be due to the limited number of studies included in the subgroup analysis, which may not accurately reflect the true effect. Third, trial sequential analysis revealed no statistically significant difference between vitamin D supplementation and no vitamin D supplementation when adjusted CIs were considered. The analysis also indicated that a larger sample size is needed to determine the true effect of vitamin D.
Regarding other outcomes in our study, vitamin D did not appear to reduce the need for mechanical ventilation and ICU admission or shorten the length of stay in the ICU and hospital. Overall, the efficacy of vitamin D in treating COVID-19 patients with vitamin D deficiency remains inconclusive. Due to the potential exclusion of vulnerable groups and the variability in the definitions of vitamin D deficiency, the interpretation of the results should be made with caution. More studies are needed to explore this further.
In 2023, Meng et al’s meta-analysis28 explored the efficacy of vitamin D in treating COVID-19. Their results showed that while vitamin D supplementation could not reduce mortality, it might be beneficial in reducing the severity of illness caused by SARS-CoV-2, particularly in vitamin D-deficient patients. Additionally, their study indicated that vitamin D supplementation could reduce the need for ICU admission. However, they did not analyse the data based on follow-up time, and new research has since been published. Our study results show that vitamin D supplementation does not reduce the need for ICU admission. Recently, a review also showed that vitamin D deficiency is linked to an increased risk of acquiring SARS-CoV-2 infection and poor COVID-19 prognosis; however, available evidence with regard to improved clinical outcomes with vitamin D supplementation is inconsistent.29 Furthermore, whether vitamin D can reduce mortality still requires further exploration.
The relationship between vitamin D and COVID-19 has been a subject of extensive research, with mixed findings regarding its efficacy in preventing or treating the disease. Observational studies that initially suggested a link between low vitamin D levels and worse COVID-19 outcomes may have been confounded by other factors such as age, comorbidities and socioeconomic status.30–34 These factors themselves are risk factors for both vitamin D deficiency and severe COVID-19, complicating the interpretation of results.35–40 A number of clinical trials have produced mixed results, with some showing no significant difference in outcomes between those receiving vitamin D supplementation and those who did not.41–45 This inconsistency suggests that vitamin D may not have a substantial impact on COVID-19 outcomes. Another possible explanation is that the design and interpretation of some studies may be problematic. It is well known that RCTs for vitamin D should be designed based on the criteria for nutrients, rather than using the pharmaceutical standards applied to drugs. As mentioned in the ‘Guidelines for optimising design and analysis of clinical studies of nutrient effects’, and as noted by Pilz et al, designing an appropriate study protocol is key to accurately assessing the impact of vitamin D on health outcomes.46 47 Therefore, optimising the study design is not only crucial for ensuring the reliability of the results, but also determines whether the evaluation of vitamin D intervention reflects its true effects.
The role of vitamin D in regulating the immune system has been extensively studied, especially in the context of viral infections.48 49 The onset and severity of COVID-19 are closely linked to the host’s immune response, and vitamin D is believed to enhance the immune system’s defence through multiple mechanisms.48 Specifically, vitamin D helps to boost the innate immune response by enhancing the function of macrophages, monocytes and dendritic cells, all of which play crucial roles in antiviral immunity.49 Additionally, vitamin D regulates T-cell differentiation, promoting cell-mediated immune responses against infections, while also suppressing excessive immune reactions, such as cytokine storms, thereby reducing the severity of the COVID-19 disease course.50
The role of vitamin D is particularly critical in the early stages of disease onset.51 Studies have shown that early intervention can significantly improve immune function and slow disease progression.21 52 For instance, supplementing vitamin D before or at the early onset of symptoms helps to promptly regulate the immune response and enhance the body’s ability to combat the virus.53 In contrast, if intervention occurs later, after symptoms have manifested or during the later stages of the disease, the effects of vitamin D may be greatly diminished.54 55 By this point, the immune system may already be in a dysregulated state, particularly under the influence of high viral loads or cytokine storms, making it difficult for vitamin D alone to quickly restore immune function.
Moreover, using high doses or active forms of vitamin D, such as 25(OH)D (calcidiol), may further enhance its therapeutic effects.56 25(OH)D is the active form of vitamin D, and it works more rapidly than regular vitamin D3.57 High-dose vitamin D interventions have shown promising clinical effects during the early stages of the pandemic.57 In particular, for high-risk patients, timely high-dose vitamin D supplementation can significantly reduce the risk of disease worsening, especially in populations with low vitamin D levels.58
Regarding high-risk groups, those at higher risk of COVID-19-related death include elderly patients, individuals with comorbidities, and patients with serum 25(OH)D concentrations below 20 ng/mL.59 The immune systems of older adults and those with chronic diseases are generally weaker, and their vitamin D levels are often lower, making them more susceptible to severe complications or death after infection.60 Additionally, studies have shown that if hospitalised patients have low vitamin D levels, their immune function is impaired, leading to more severe clinical outcomes.59 Therefore, for these high-risk groups, timely and appropriate vitamin D intervention could be a critical measure to reduce the mortality rate and severity of the COVID-19 disease course.61
However, it is important to note that vitamin D supplementation may also have potential adverse effects, such as hypercalcaemia and hypoparathyroidism, particularly when taken in excessive doses.62 63 These adverse effects should be considered when evaluating the overall benefits and risks of vitamin D supplementation, especially in vulnerable populations.
In summary, vitamin D supplementation has the potential to reduce the incidence, severity and mortality of COVID-19. However, its effectiveness depends on multiple factors, particularly the timing and dosage of intervention. Moreover, factors such as the economic status, sex and age of patients may serve as effect modifiers that could influence the outcomes, which were not thoroughly analysed in our study. Future research is needed to further clarify the optimal timing and dosage for vitamin D intervention, and whether personalised treatment plans based on patients’ underlying conditions and vitamin D levels are necessary. Furthermore, during the pandemic, it is important to encourage high-risk populations (such as older adults and individuals with chronic diseases) to maintain adequate vitamin D levels to enhance immunity and improve the body’s ability to combat COVID-19.
In this study, we found significant differences in the definition of ‘vitamin D deficiency’ across studies, which may introduce selection bias. Some studies defined deficiency as a serum vitamin D level below 30 ng/mL, while others used 20 ng/mL, which could lead to overdiagnosis or underdiagnosis of vitamin D deficiency. Specifically, for elderly patients, a higher threshold (eg, 25 ng/mL) might result in their exclusion from studies, thus affecting the study conclusions. We recommend that future research adopt standardised definitions of vitamin D deficiency and adjust the criteria based on patient characteristics (such as age, sex and comorbidities) to reduce potential selection bias and misdiagnosis.
Moreover, the variability in vitamin D categorisation may impact the assessment of treatment efficacy. Due to the inconsistent standards for defining vitamin D deficiency across studies, some studies may have underestimated the effect of vitamin D on treatment outcomes. To improve the accuracy of results, we suggest that future studies consider individualised vitamin D deficiency criteria based on different population characteristics and further explore the impact of these criteria on treatment efficacy, ensuring that all patients with true vitamin D deficiency are included in the analysis.
However, our study also has other limitations. First, the number of studies included is relatively small, with only nine RCTs and small sample sizes. Second, although there was no significant statistical heterogeneity, clinical heterogeneity among the studies cannot be ignored. The severity of patients’ diseases and the frequency and dosage of vitamin D supplementation varied among the studies. To address this, we conducted a subgroup analysis and found that vitamin D supplementation did not reduce mortality in different subgroups. Third, there is a potential risk of publication bias in our study. Although Egger’s test did not show significant publication bias, the number of studies included in our analysis is relatively small, so caution is still needed when interpreting the risk of publication bias. Finally, although our conclusions suggest that vitamin D supplementation may reduce mortality, sensitivity analysis revealed that the conclusions are not reliable. Therefore, more high-quality research is needed in the future to further explore the role of vitamin D supplementation in vitamin D deficient COVID-19 patients.
Conclusion
This study suggested that vitamin D supplementation might have reduced mortality during follow-up, but no significant difference was observed in mortality at 28 days. Additionally, vitamin D supplementation did not significantly improve the need for mechanical ventilation, ICU admission rate or reduce hospital and ICU length of stay. While these results indicated that vitamin D might have had some impact on mortality in COVID-19 patients with vitamin D deficiency, the findings should be interpreted cautiously due to variations in the studies and potential selection biases. Future research should focus on high-quality clinical trials, particularly those considering individual differences, study design and follow-up duration, to draw more reliable and consistent conclusions.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We would like to give our sincere gratitude to the reviewers for their constructive comments.
References
Footnotes
Contributors BH is the guarantor of this study. LZ, PB, MQ and BH: proposed the design, searched the literature, collected, analysed and interpret the data, and wrote the report; LZ, XZ, YZ and XL searched and collected the literature; LZ, YZ, XZ, XL and BH analysed and interpreted the data.
Funding The work was supported by the Hunan Provincial Education Commission Foundation (20A056, 23A0664, 24A0683); the Hunan Provincial Health Commission Foundation (no. 202112041226, D202302088596); the Innovation and Entrepreneurship Education Base of Public Health and Preventive Medicine (Hunan Education Bureau Notice 2019, no. 333-93) and the funding by young backbone teachers of Hunan province training programme foundation of Changsha Medical University (Hunan Education Bureau Notice 2021, no. 29-26, Hunan Education Bureau Notice 2023, no. 318-26).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.