Article Text
Abstract
Objectives Penpulimab is a novel programmed death-1 (PD-1) inhibitor that has been approved in China for use in combination with chemotherapy as a first-line treatment for locally advanced or metastatic squamous non-small cell lung cancer (sq-NSCLC). However, the cost-effectiveness of this treatment in China remains to be determined. In this study, we aimed to assess the cost-effectiveness of penpulimab combined with paclitaxel and carboplatin for metastatic sq-NSCLC.
Design Based on the AK105-302 trial (NCT03866993), a Markov model was created to evaluate the disease progression of metastatic sq-NSCLC patients over 10 years. The model included progression-free survival, progressive disease and death. The utility values were derived from published literature. Sensitivity studies were used to assess the robustness of the model outputs.
Setting The Chinese healthcare system perspective.
Participants A hypothetical Chinese cohort of patients with locally advanced or metastatic sq-NSCLC.
Interventions Penpulimab plus chemotherapy versus chemotherapy.
Primary outcome measure Costs, quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER).
Results Compared with the chemotherapy alone group, the cost of penpulimab plus chemotherapy increased by US$3717.72, with an increase of 0.43 QALYs. The ICER was US$8625.78/QALY, which was well below the willingness-to-pay threshold of US$38 052/QALY. This demonstrated higher cost-effectiveness benefits, as confirmed by the sensitivity analysis results.
Conclusions Under the Chinese health system, penpulimab plus paclitaxel and carboplatin is cost-effective for metastatic sq-NSCLC patients and can be used as an economical and effective treatment option.
- Health economics
- ONCOLOGY
- PUBLIC HEALTH
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study used a Markov model to evaluate the cost-effectiveness of penpulimab combined with paclitaxel and carboplatin for metastatic sq-NSCLC from the perspective of the Chinese healthcare system.
A large, multicentre, randomised clinical trial (AK105-302, NCT03866993) was used as the basis for this study.
Sensitivity analyses were used to explore the stability of the model and compare the effects of different factors on the outcomes.
The long-term benefits of this treatment remain to be explored.
The health utility values were derived from relevant literature, which may have been unrepresented.
Introduction
In recent years, lung cancer has emerged as the most prevalent malignant tumour type globally, with an increasing incidence and mortality rate. The latest report by the International Agency for Research on Cancer (IARC) has indicated that the global incidence of new lung cancer cases in 2022 reached 2.48 million, representing 12.4% of all new cancer cases and establishing lung cancer as the most prevalent cancer type worldwide.1 Approximately 80–85% of all lung cancer cases are non-small cell lung cancer (NSCLC),2 with squamous histological subtypes accounting for nearly 25%.3 Conventional platinum-based two-agent chemotherapy has historically been the standard therapeutic option for squamous NSCLC (sq-NSCLC) patients. However, this subtype has a low frequency of sensitive mutations and poorer prognosis than lung adenocarcinoma.4 5 Consequently, the development of programmed death-1 (PD-1) and PD-ligand 1 (PD-L1) inhibitors has provided alternative approaches for treating sq-NSCLC.
A review of clinical studies has demonstrated the efficacy of using a PD-1/PD-L1 inhibitor plus chemotherapy to prolong sq-NSCLC patient survival.6–8 In addition, the Chinese Society of Clinical Oncology (CSCO) guidelines recommend that the first-line therapeutic option for metastatic sq-NSCLC be PD-1/PD-L1 inhibitors, either alone or in combination with chemotherapy.9 Nevertheless, the relatively higher cost of imported PD-1/PD-L1 inhibitors and the high cost of prophylaxis have somewhat limited the widespread use of this class of drugs, emphasising the importance of developing domestic PD-1/PD-L1 inhibitors.
Penpulimab, a novel PD-1 inhibitor that was developed in China, belongs to the IgG1 subtype of monoclonal antibodies and features a uniquely modified fragment crystal structure.10 In January 2023, penpulimab was approved by the Chinese National Medical Products Administration (NMPA) in combination with paclitaxel and carboplatin for locally advanced or metastatic sq-NSCLC. The results of a clinical trial (AK105-302) evaluating the efficacy of penpulimab plus chemotherapy for metastatic sq-NSCLC were used to inform the relevant indication.11 The results demonstrated that penpulimab plus chemotherapy treatment significantly prolonged the progression-free survival (PFS) of patients compared with the chemotherapy alone group (7.6 months vs 4.2 months, respectively). Additionally, the risk of disease progression or death was reduced by 56%. Furthermore, the treatment had a favourable tolerability profile, with no significant increase in adverse events (AEs) occurring during the trial period. Although penpulimab in combination with chemotherapy has demonstrated promising clinical benefits, the economic implications of its use in China remain uncertain. Therefore, in this study, we aimed to examine the cost-effectiveness of penpulimab plus chemotherapy in Chinese metastatic sq-NSCLC patients.
Methods
Target population and interventions
The target population of AK105-302 was locally advanced or metastatic sq-NSCLC patients in China. Participants were randomised (1:1) to receive penpulimab or placebo in combination with carboplatin (area under curve 5 mg/mL/min) plus paclitaxel (175 mg/m2) intravenously on day 1 of every 3 weeks for a total of four cycles, followed by penpulimab or placebo as maintenance therapy. We assumed a body surface area of 1.72 m² and creatinine clearance rate of 70 mL/min.12 13
Model structure
Using the AK105-302 trial, we developed a Markov model to analyse the cost-effectiveness of penpulimab plus paclitaxel and carboplatin as first-line treatment. Three main clinical states were identified: PFS, progressive disease (PD) and death. The dosing regimen of the clinical trial was employed to define the cycle, which was 21 days. The duration of the trial was set to 10 years, with the assumption that all participants were in the PFS state at the start of the model and transitions between states were irreversible, as shown in figure 1. The main output metrics were the total cost and quality-adjusted life years (QALYs), with a discount rate of 5% applied to evaluate the economy of the treatment scheme. According to the 2023 National Economic and Social Development Statistics Bulletin,14 three times the gross domestic product (GDP) per capita (US$38 052) was set as the willingness-to-pay (WTP) threshold.
Markov models. PD, progressive disease; PFS, progression-free survival.
Transfer probability
The survival data were acquired from the AK105-302 trial with the Engauge Digitizer software, which was used to extract the raw survival curves of the two groups, reconstruct the individual data, and fit parameter distributions using R studio. The parameter distributions used in this study included Weibull, Exponential, Log-logistic, Log-normal and Gompertz. The fitting distributions were identified using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) values in conjunction with examining the fitted graph for each distribution. The AIC and BIC values of the fitting model are shown in the online supplemental table. The relevant parameters are presented in table 1.
Supplemental material
K-M curve optimum fitting distribution and parameters
Cost and utility values
Considering the Chinese healthcare system and the situation of the AK105-302 clinical trial, we only evaluated the direct medical costs, including drug costs, medical management, routine follow-up, AEs, and best supportive care costs. Drug prices were obtained from public databases.15 Medical management costs included the costs of consultations, injections, hospitalisation and nursing care and were set at one visit per treatment cycle. After disease progression, the chemotherapy group was transitioned to receive penpulimab monotherapy. The AK105-302 trial did not provide details on post-progression medications and ratios in the experimental group under the Clinical Guidelines for the Diagnosis and Treatment of Lung Cancer published by CSCO 2023.16 Therefore, we assumed that the experimental group would receive second-line treatment after disease progression, including best supportive care and chemotherapy (docetaxel). The costs obtained from the literature were adjusted to 2023 prices using the annual growth rate of the Chinese Consumer Price Index for Healthcare and Personal Goods. In accordance with the 2020 edition of the China Pharmacoeconomic Evaluation Guidelines,17 only AEs of grade ≥3 (incidence >5%) were included, assuming that each AE occurred only once. The model inputs are shown in table 2.
Model inputs
Sensitivity analysis
To test the stability of each parameter on our cost-effectiveness model, one-way deterministic sensitivity analyses (DSA) and probabilistic sensitivity analyses (PSA) were conducted. For the one-way DSA, PFS and PD status utility values were analysed with the 95% CIs of the literature data. Other costs and AE incidence were analysed with 30% of the basic value as the upper and lower limits, with tornado diagrams used for the output results. Second-order Monte Carlo simulations were performed for the distribution form of each parameter. The results were graphically illustrated using cost-effectiveness scatter plots and acceptability curves.
Scenario analysis
Because penpulimab had not yet been listed in the National Health Insurance Drug List (NRDL) for metastatic sq-NSCLC, we assumed that the price of penpulimab was between 0.5 times and 2 times in the NRDL.
Patient and public involvement
Patients and/or the public were not involved in this study.
Results
Base-case analysis
The results showed a cost increase of US$3717.72 and an effectiveness increase of 0.43 QALYs for the penpulimab group compared with the chemotherapy alone group, with an incremental cost-effectiveness ratio (ICER) of US$8625.78/QALY (table 3). This was well below the WTP threshold of US$38 052/QALY.
Base-case results
One-way deterministic sensitivity analyses
The one-way DSA for the penpulimab and chemotherapy groups are presented in figure 2. The results showed that the main parameters affecting ICER were the discount rate and penpulimab cost, with the discount rate having the greatest impact on the results. Because the relevant parameters moved up and down within the designated range, the ICER consistently remained below the set WTP threshold, thereby corroborating the base-case analysis findings and indicating that the model was relatively stable.
Tornado analysis of penpulimab plus chemotherapy versus chemotherapy. ICER, incremental cost-effectiveness ratio; PD, progressive disease; PFS, progression-free survival; QALY; quality-adjusted life year.
Probabilistic sensitivity analyses
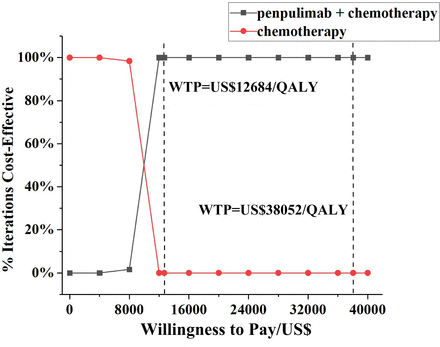
The PSA scatter plot shows the incremental cost and effect data derived from 1000 Monte Carlo simulations. As illustrated in figure 3, the scatter plot demonstrated that the values were all below the WTP threshold in the first quadrant when WTP was set at 1×GDP per capita. Furthermore, the acceptability curves demonstrated that the penpulimab group exhibited a pronounced advantage over the chemotherapy group at WTP thresholds spanning from US$12 684/QALY to US38 052/QALY (1×to 3×GDP per capita), with a 100% probability of cost-effectiveness (figure 4).
The scatter plot diagram showed the probability at the current WTP threshold. QALY, quality-adjusted life year; WTP, willingness-to-pay.
Cost-effectiveness acceptability curves for penpulimab plus chemotherapy versus chemotherapy. QALY, quality-adjusted life year; WTP, willingness-to-pay.
Scenario analysis
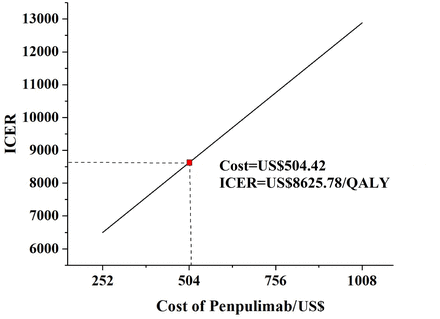
Figure 5 shows the impact of penpulimab cost on the ICER. The cost of penpulimab varied between US$252 and US$1008 (0.5–2 times the current price). As the cost of penpulimab increased, the ICER also increased. In all cases, the ICER was below the WTP threshold (US$38 052/QALY).
Results of scenario analysis. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Discussion
In the AK105-302 clinical trial, penpulimab plus chemotherapy demonstrated significant advantages over chemotherapy alone for locally advanced or metastatic sq-NSCLC. The median PFS was prolonged by 3.4 months, with a high 30 month overall survival rate of 51.6%, and the treatment regimen was well tolerated. From this study, the NMPA approved penpulimab plus paclitaxel and carboplatin for the first-line treatment of locally advanced or metastatic sq-NSCLC. In 2021, penpulimab was approved for the treatment of relapsed or refractory classical Hodgkin’s lymphoma in patients who had received at least two systemic chemotherapies.10 18 This approval provides Chinese NSCLC and lymphoma patients with additional therapeutic options.
As the fifth domestically produced PD-1 inhibitor, penpulimab has demonstrated promising clinical efficacy and safety. However, its cost-effectiveness has not been explored with pharmacoeconomic evidence. Here, we addressed this unmet need for a penpulimab economic evaluation. For the first time, we evaluated the cost-effectiveness of penpulimab plus chemotherapy for metastatic sq-NSCLC in China. Base-case analysis indicated that the penpulimab group displayed a greater number of QALYs, although this was accompanied by a corresponding increase in cost. When compared with the chemotherapy group, the ICER was US$8625.78/QALY, which was well below the WTP of US$38 052/QALY. The sensitivity analysis indicated that the cost-effectiveness of the penpulimab group was likely to be nearly 100% at this threshold level.
In recent years, the state has actively promoted the development and application of domestically produced anticancer drugs. This initiative, coupled with a national drug price negotiation system, has resulted in a notable reduction in drug costs.19 These measures have facilitated a gradual increase in the use of domestically manufactured anticancer drugs in the Chinese market, offering significant benefits to cancer patients in the country. Currently, penpulimab in combination with chemotherapy is not included in the National Reimbursement Database for metastatic sq-NSCLC. Therefore, we considered a broad price range for penpulimab to provide a rough assessment of the acceptability of treatment access for patients. The results showed that the ICER increased with rising penpulimab costs, which were all below the WTP threshold. Overall, the economic results indicated that this drug offers certain cost-effectiveness advantages, which are anticipated to become more pronounced after it is covered by health insurance. This study provides evidence that supports the wider use of domestically manufactured anticancer drugs.
However, this study has some limitations. First, because the health utility values were not published with the results of the AK105-302 study, we choose to refer to data from relevant Chinese NSCLC patients. This may have introduced bias into the results. Furthermore, the long-term benefits of penpulimab plus chemotherapy treatment for sq-NSCLC remain unclear. The model estimated subsequent treatment and long-term efficacy. However, the extrapolation of data from the study could have introduced bias by over- or underestimating the long-term survival outcomes. Next, we did not consider grade 1 or 2 AEs. Despite being relatively mild, these AEs can still impact the total cost. Additionally, potential changes in the clinical efficacy of penpulimab plus chemotherapy compared with chemotherapy alone for sq-NSCLC stratified by the PD-L1 tumour proportion score were not considered in this study. More detailed subgroup data will be collected in the future to explore the economic benefits. Lastly, the research perspective, modelling approach and selection of parameter inputs may have also influenced the generalisability of our penpulimab cost-effectiveness analysis. Despite these limitations, the conclusions of our sensitivity analyses corroborated those of the base-case analysis that penpulimab plus chemotherapy represents a cost-effective treatment approach in China.
Conclusion
In summary, penpulimab plus chemotherapy proves to be more cost-effective than chemotherapy alone for metastatic sq-NSCLC. Furthermore, this treatment regimen is a viable and cost-effective therapeutic option within the Chinese healthcare system.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Footnotes
Contributors LW, NL, CY and YG were involved in the study conceptualisation. LW and NL completed the data collection and analysis. LW wrote and edited the draft. XH provided the related resources and methods. RD is responsible for the overall content as the guarantor. All authors approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.