Article Text
Abstract
Introduction The immunological composition of breastmilk has gained research interest as breastfeeding has been persistently correlated with improved health outcomes in children. Immune cells, also known as leucocytes, are key components of the body’s immune system, but they remain understudied in breastmilk. The relevance of breastmilk leucocytes for breastfeeding-mediated immune benefits remains controversial. To identify the current state-of-the-art on breastmilk leucocyte research, unearth knowledge gaps and propose research priorities, a scoping review is necessary.
Methods and analysis This scoping review will address the general question of what is known about leucocytes in human breastmilk. The development of this scoping review protocol adhered to the recommendations set forth by the Joanna Briggs Institute guidelines. Peer-reviewed research articles published in English, French or Spanish will be eligible for inclusion in the scoping review. The initial literature search was conducted in January 2024 within the Medline, Embase, Cochrane Central and BVS databases.
Ethics and dissemination This review does not require ethics approval. Our dissemination strategy includes peer-review publication and presentations at conferences and to relevant stakeholders.
Registration details This protocol was registered in Open Science Framework (available at: https://osf.io/kwfsy) on 19 February 2024.
- Paediatric infectious disease & immunisation
- IMMUNOLOGY
- Review
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This scoping review represents the first systematic exploration of the literature on leucocytes in human breastmilk, underscoring the novelty of this research.
We propose a novel system for quality appraisal of the literature on qualitative and quantitative characterisation of breastmilk leucocytes.
We will focus our analysis on peer-reviewed literature only, deposited in four databases, and published either in English, French or Spanish. Therefore, some relevant publications indexed in other places or in a different language may be missed.
Introduction
Breastfeeding is encouraged by multiple agencies worldwide to promote health in both infants and mothers. Beyond aiding development through nutrition, breastmilk provides maternally derived immune factors to suckling infants, including antibodies, cytokines and growth factors.1–3 These components protect infants from infectious diseases, sustain the maturation of the intestine and promote the establishment of the commensal microbiota.4 5
In addition to soluble factors, live immune cells, also called leucocytes, have been found in breastmilk. The composition of breastmilk leukocytes (BreLeuk) is dynamic and varies together with mother and infant health, maternal body mass index and gestational age at birth, among other conditions impacting the mother-infant dyad.5–7 Along the process of lactation, from the development of the mammary glands until involution, leucocytes of multiple lineages including lymphocytes, granulocytes, monocytes and macrophages travel from peripheral tissues to the maturating mammary gland by blood and lymph, reside in situ and are found in breastmilk.8–10 The exact process regulating the presence of leucocytes in breastmilk remains elusive.
A variety of functions or roles have been proposed for BreLeuk. For instance, B lymphocytes produce the antibodies responsible for the passive immunity provided by breastmilk. While antibodies have been shown to travel through the transcytosis route from mammary tissue into breastmilk, antibody-producing B lymphocytes have also been described in breastmilk.11 12 In mice, BreLeuk could survive the digestion process and migrate to various tissues from suckling pups, effectively creating microchimerism.13 14 Additional animal studies have described a specific T lymphocyte subpopulation with effects on the intestinal microbiota and the local immune system that may persist across multiple generations, potentially through factors transferred through breastmilk.15 Unique lactation-induced macrophages were recently described in mouse breastmilk to also impact the microbiota establishment.16 BreLeuk could directly participate in microbiota and pathogen regulation in situ through their multiple functions, for example, phagocytosis.5
Therefore, scattered information has been documented on BreLeuk in a variety of contexts, and BreLeuk may play relevant roles directly in the milk within the mammary gland or once ingested in the infant’s intestine. Available reports are not comprehensive, focusing on a limited number of leucocyte lineages at a time and/or maternal-infant conditions. Additionally, a limited number of studies pertain to the human species. The heterogeneity of breastmilk composition during lactation further complicates painting a comprehensive picture of the current landscape in human BreLeuk science.8 Given the probable relevance of BreLeuk for promoting health in suckling infants, we propose here a protocol for a scoping review to systematically review the scientific literature reporting on human BreLeuk.
A search in PROSPERO, Open Science Framework, Medline, Embase, Cochrane Database of Systematic Reviews and BVS was conducted, and no review on this topic was found at the time of writing (January 2025). The objective of this scoping review is to identify and organise the available information from the peer-reviewed original scientific literature about maternal leucocytes (including but not restricted to proportions, concentrations, subsets and described functions) in human colostrum/transitional milk and mature milk.
Methods and analysis
Study design
The aim of this protocol is to answer the research question formulated as:
‘What is known about human breastmilk leucocytes present in human milk during lactation?’
The purpose of this review is to synthesise relevant qualitative and quantitative data from experimental research studies reporting on human BreLeuk. We will select peer-reviewed research articles containing information on any human BreLeuk lineage and report the information together with its associated metadata. The proposed scoping review protocol has been developed following the Joanna Briggs Institute (JBI) guidelines for scoping reviews17 and has been registered with the Open Science Framework (https://osf.io/kwfsy). In addition, this protocol complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement for protocols, for all items that apply to scoping reviews (online supplemental table 1).18 We formulated the search strategy based on our research question, following the population, concept and context framework as shown in table 1.
Supplemental material
Population-concept-context
Sources
The four selected databases for article retrieval are Medline (OVID), Embase, Cochrane Central Register of Control Trials and BVS.
The relevant studies considered for this review will exclusively be peer-reviewed, published primary research articles. Study designs eligible encompass experimental and quasiexperimental, including randomised and non-randomised controlled trials, and analytical observational studies (prospective case-control studies and analytical cross-sectional studies). Case series, individual case reports and descriptive cross-sectional studies are also eligible. Excluded studies include opinions, reviews, grey literature, conference abstracts and books. Articles included in the final output must satisfy the criteria described in table 2. If a relevant article is not freely available online, support will be sought from the participants' institutional library services and by contacting the article’s corresponding author(s).
Inclusion and exclusion criteria
Search strategy
On 25 January 2024, an exploratory search was undertaken by an expert librarian (SCD), restricted to the Medline database. The strategy involved applying keywords and medical subject headings (MeSH) terms that associate with relevant key concepts, together with Boolean terms ‘AND’ and ‘OR’. The MeSH terms used were the following: ‘Milk, Human’, ‘Lactation’, ‘Breast Feeding’, ‘Colostrum’, ‘Leukocytes’, ‘Lymphocytes’, ‘Killer Cells, Natural’, ‘Monocytes’, ‘Dendritic Cells’, ‘Granulocytes’, ‘Neutrophils’, ‘Eosinophils’, ‘Basophils’, ‘Natural Killer T-Cells’, ‘B-Lymphocytes’. The free terms used for this initial search were: Human milk, mature milk, breast milk, Maternal milk, transitional milk, Foremilk, Hindmilk, breastmilk, breastfeed*, ‘Breast feed*’, Maternal transfer, Colostr*, Lactation, leukocyte*, lymphocyte*, B-Lymphocytes, myeloid, lymphoid, ‘Natural Killer’, ‘NK Cells’, Monocytes, Macrophage*, ‘dendritic cell*’, ‘White blood cells’, ‘White blood Corpuscle’, ‘Immun* cells’, Granulocytes, Neutrophil*, Eosinophil*, Basophil, ‘B cells’, ‘T cells’. The search was restricted to the human species; however, the exploratory search retrieved a very large proportion of non-human animal studies, prompting iterations to the search strategy. Nine known relevant publications proposed by BreLeuk expert team members were confirmed to be present in the search output, which validated its scope.
The final search was performed from inception to 31 January 2024 in the specified databases using the search string detailed in (online supplemental appendix 2). The strategy was then adapted by the expert librarian to the three additional databases (online supplemental appendices 3–5).
Supplemental material
Selection of sources of evidence
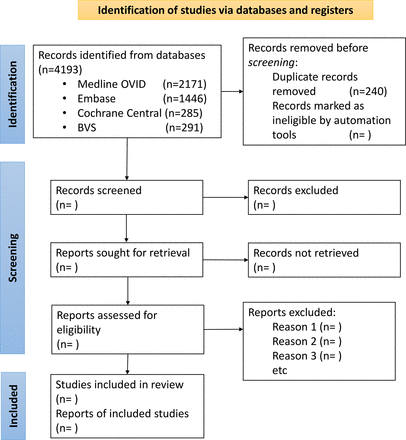
A total of 4193 articles were originally retrieved from the four databases and loaded to the Rayyan platform,19 where 291 duplicates were deleted, giving a final selection of 3953 articles available for screening. The first screening process will be conducted blindly and independently by three researchers from the team (BJL, CAGA and ESS), using Rayyan. Screening will involve scrutiny of titles and abstracts of each article according to the selection criteria described in table 2. Discrepancies in screening will be resolved by consensus after a thorough revision involving an additional topic expert from the team (MEGB). The second screening will be based on the full text version of each article to confirm relevance and will be performed by three team members (BJL, CAGA and ESS), independently. A preliminary flowchart of this process is described in figure 1, which follows the PRISMA for Scoping Reviews (PRISMA-ScR) guidelines.20
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Charting the data
Once the final list of articles to be included in this scoping review is complete, the full text versions of the articles will be scrutinised to collect relevant metadata. This will be done by three team members (BJL, CAGA and ESS) independently, and the data will be charted automatically into an Excel document using Google Forms. Advantages of using Google Forms for the data charting process include compulsory filling of information, leading to no empty fields that may cause confusion between unavailable data and the answer ‘No’. It also allows the team to be working simultaneously, blindly and unambiguously on the same publication. The Google Forms questionnaire elaborated to collect information for the scoping review is described in box 1. While various questionnaire items may seem redundant (eg, Q24 and Q25), this will allow a more robust description of the studies (in the previous example, ‘colostrum’ labelling has been used to describe breastmilk collected <2 days post partum in some studies, but until <7 days post partum in others7 21–23).
Metadata: charting items
Publication information
Q1. Complete publication title
Q2. Authors
Q3. Nomenclature of the article from our database
Q4. Language
Q5. Keywords used
Study design Please describe the parameters of the study in more detail (eg, follow-up length, intervention, etc…)
Q6. What was the overall aim of this study?
Q7. Study type (observational study, intervention, cross-sectional, longitudinal, retrospective, prospective and/or other)
Q8. Complete cohort size (number of mothers included in the study)
Q9. Subgroup description (eg, obese vs lean) and size
Q10. Patients' dropout (only in clinical trials) --> If not relevant: NA, if relevant, add number of dropouts
Q11. Are infants also considered/described in this work?
Demographic and clinical data of the cohort participants
Q12. Nationality of the mothers
Q13. Age range of mothers (eg, 18–38)
Q14. Health status of the test group of mothers (preeclampsia, gestational diabetes, COVID-19, HIV and/or other)
Q15. Additional information about mothers (ethnicity, socioeconomic background, body mass index, recruitment from a special group, etc…)
Q16. Recorded maternal medications or supplements of any kind (even outside of an intervention)
Q17. If infants were considered, what specific health status is included? (healthy, term, preterm, infections and/or other)
Q18. If infants with infections were considered, which pathogen(s) is/are involved?
Q19. If infants with non-infectious diseases were included, describe which ones
Q20. Delivery method (vaginal delivery or C-section)
Q21. Gestational age (if available, in weeks)
Q22. Maternal food intake? No or yes with details.
Q23. Number of deliveries of the mother
Milk sampling information
Q24. Milk type description (colostrum, transitional milk, mature and/or other)
Q25. Timing of sample collection postpartum (in number of days)
Q26. Information on exact timing of collection within the day (eg, morning, pre-infant feeding or post-infant feeding, 30 min into feeding, etc…)
Q27. Methodology for collection (manual vs automatic pump, cleaning of the area, discard first drops, etc…)
Q28. Range of volumes collected. Or exact volume if the case may be
Q29. Processing method (eg, length of time between collection and processing, length of processing post collection (could affect neutrophil measurements, leucocyte activation, etc…), dilutions (in what buffer/medium?), centrifugation speed? Discard supernatant? Enrichment of populations (by density gradient, magnetic beads, etc…?), temperature of processing)
Q30. Long-term storage temperature
Q31. Other bioactives measured from milk samples?
Q32. Any confounding aspects in the methodology details?
Leucocyte analysis*
We have eight defined questions (Q34-Q41) aimed at gathering as much information as possible about each analysed leucocyte. Reactives Q41-Q121 correspond to the repetition of these eight questions, available for as many leucocyte subtypes reported in each publication.
Q33. All immune cell populations of interest in this study
Q34. Immune cell population of interest in this section (first)
Q35. Description of the regulation of the phenotype of that cell in the paper
(expression of surface molecules, cytokines, modification of function)
Leucocyte #1
Q36. Type of analysis performed (flow cytometry, microscopy, transcriptomic analysis and/or other)
Q37. In the case of flow cytometry analyses, indicate what gating strategy was used to identify each population.
Q38. In the case of flow cytometry specify all that apply: (use/not use of viability marker, gating/not gating on single cells, use/not use of Fluorescence Minus One (FMO) controls, gating strategy provided/not provided as a figure, clear/unclear parental population description)
Q39. Additional methodology details
Q40. Methodology and results (identity or phenotype modification (concentrations, phenotype, changes between groups/treatments, other relevant results on leucocytes in milk)
Q41. Additional information of interest outside of current categories
Q42-Q121. Repetition of the questions Q33 to Q41 for the different leucocytes that are described in the studies.
Analysis and presentation of results
We will adhere to the PRISMA-ScR guidelines to report the findings from this study.19 The full list of included articles will be provided as part of the publication. The results of the scoping review will be categorised according to relevant themes including but not restricted to study demographics, methodologies and leucocyte subtype. These results will be presented as evidence maps, such as tables and diagrams, and narrated in text.
Metadata will be summarised to provide a comprehensive overview of the field, and specific relevant findings will be detailed, including frequency and concentration of leucocyte subtype in milk.
Subthemes regarding the heterogeneity of populations (demographics) or maternal health status may emerge and will be described and discussed, as relevant.
Evidence quality appraisal
We anticipate the retrieved articles to be mostly observational studies. Given the unavailability of a quality assessment tool for the data in our scoping review, we developed a tool based on the JBI Critical Appraisal Checklist,24 merged with the adaptation of the Cochrane risk of bias tool, done by Wylde et al in 2017.25 Our tool will grade each included paper for risk of bias, from 0 (no identified risk) to 1 (low risk, minor inconsistencies) to 2 (high risk, major inconsistencies), in answering the five questions below:
Were the criteria for mother inclusion in the study clearly defined?
Were technical confounding factors identified? (eg, viability marker not used for flow cytometry, etc.)
Were quantitative data provided for all analysed leucocytes declared in methods?
Were the methods described in sufficient detail to allow reproducible experiments?
Other bias.
Patient and public involvement
Patients and the public were not involved in this work.
Discussion
Breastfeeding is a time-restricted opportunity to positively impact short-term and long-term health. As research moves from empirical evidence to mechanistic explanations for the known benefits of breastmilk, BreLeuk emerges as enigmatic participants in breastmilk-mediated health. The scoping review proposed in this protocol will help summarise what is known about leucocytes in human breastmilk, providing quantitative and qualitative evidence to help direct future research efforts.
Ethics and dissemination
This review does not require ethics approval. Our dissemination strategy includes peer review publication, presentations at conferences and to relevant stakeholders.
Ethics statements
Patient consent for publication
Acknowledgments
The authors acknowledge all funding bodies and administrative support from Tecnológico de Monterrey, Karolinska Institutet and CONAHCyT (Mexico).
References
Footnotes
X @YepesNunez, @MarionBrunck
CAG-A and BJ-L contributed equally.
Contributors CAGA and BJL produced the preliminary search strategy, wrote and edited the protocol and developed the data charting form. SCD, JJYN and AML produced and edited the exploratory search and final search strategies and produced and edited the manuscript. MEGB and ESS conceived the study, wrote and edited the manuscript and MEGB additionally secured research funding. All authors approved the final version of the manuscript. MEGB is the guarantor of this work.
Funding This research was supported by the Institute for Obesity Research at Tecnológico de Monterrey and a research grant from the Centro de Primera Infancia of Tecnológico de Monterrey (TRIADA), the research grant CF-2023-G-990 from Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCyT) and the postgraduate scholarships CVU 1315999 and CVU 1315817 from CONAHCyT.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.