Article Text
Abstract
Introduction Atelectasis is a common postoperative complication in patients with obesity, contributing to respiratory insufficiency, pneumonia and poor clinical outcomes. Studies have shown that driving pressure (DP)-guided individualised positive end-expiratory pressure can improve respiratory mechanics and oxygenation, while also reducing the incidence of atelectasis and other postoperative pulmonary complications (PPCs). However, the effect of this ventilation strategy on patients with morbid obesity remains unclear. The trial presented here aims to use lung ultrasound to evaluate the effect of DP-guided individualised positive end-expiratory pressure (PEEP) on postoperative atelectasis in patients with obesity undergoing bariatric surgery.
Methods and analysis This single-centre, randomised, controlled, single-blind study will enrol 52 participants with morbid obesity scheduled for laparoscopic bariatric surgery from 1 March 2024, to 30 April 2025. They will be randomly assigned in a 1:1 ratio to one of two groups: (1) the DP group, where participants will receive dynamic individualised PEEP guided by DP and (2) the fixed PEEP group, where participants will receive a PEEP of 8 cmH2O. The primary outcome is the lung ultrasound score 30 minutes after extubation. Secondary outcomes include the lung ultrasound score on postoperative day 1, the incidence and severity of PPCs within 3 days after surgery, the intraoperative partial pressure of arterial oxygen, DP, static lung compliance, mechanical power, the incidence of hypotension during titration and the dosage of vasoactive drugs.
Ethics and dissemination This study has been approved by the Ethics Committee of The First Affiliated Hospital of Shandong First Medical University (YXLL-KY-2023(144)). The trial results will be published in peer-reviewed journals and at conferences.
Trial registration number https://clinicaltrials.gov/; NCT06181279.
- Obesity
- Adult anaesthesia
- GASTROENTEROLOGY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study is a well-designed randomised controlled trial providing good intergroup comparability and avoiding selection bias.
This study will evaluate atelectasis using lung ultrasound, which has the advantages of simplicity, safety, reproducibility and non-radiation.
This study will conduct three titrations to achieve dynamic individualised positive end-expiratory pressure (PEEP), rather than using a single titrated PEEP throughout the surgery.
This is a single-centre study, thus, the external generality is limited.
Introduction
General anaesthesia in patients with morbid obesity (MO) causes significantly more atelectasis compared with non-obese patients.1 Oxygen reserve, respiratory compliance and functional residual capacity are reduced in obese patients due to the accumulation of thoracic and abdominal adipose tissue.2 In addition, the use of pneumoperitoneum during bariatric surgery increases the risk of pulmonary atelectasis.3 4 Pulmonary atelectasis causes varying degrees of impairment of gas exchange and respiratory mechanics intraoperatively and postoperatively. In severe cases, lung collapse can contribute to postoperative respiratory insufficiency, pneumonia and worse clinical outcomes.2
Positive end-expiratory pressure (PEEP) is a strategy that helps to keep the alveoli open and to prevent postoperative pulmonary atelectasis.5 However, considering the individual differences in patients, a fixed PEEP is not suitable for all patients. Therefore, optimal PEEP should be set to meet individualised needs. Studies6 7 suggest that individualised PEEP, which avoids both alveoli overdistension and collapse, offers significant advantages over fixed PEEP in improving respiratory mechanics and reducing the incidence and severity of pulmonary atelectasis.
Driving pressure (DP) is demonstrated to be the only significant mediator associated with postoperative pulmonary complications (PPCs) in ventilated patients.8 Tharp et al 9 observed that patients with MO exhibited higher DP. As a simple and practical method for titrating PEEP and reducing DP, DP-guided individualised PEEP has gained increasing attention in clinical research and has provided favourable lung protection during various types of surgery.10–13 However, few relevant studies explored the effect of DP-guided individualised PEEP in patients with MO. Therefore, this study intends to explore the effect of DP-guided individualised PEEP on PPCs in patients with MO undergoing bariatric surgery and to provide a reference for clinical application of this ventilation strategy.
Methods
Objectives and design
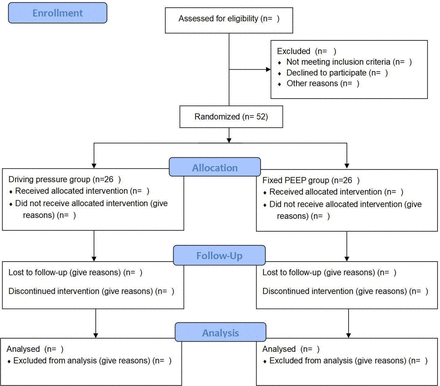
This single-centre, randomised, controlled, single-blind study has been registered with ClinicalTrials.gov (NCT06181279) before enrolment. It will be conducted at the First Affiliated Hospital of Shandong First Medical University, Jinan City, Shandong Province. From 1 March 2024 to 30 April 2025, a total of 52 participants will be assigned to the DP group or the fixed PEEP group randomly (see Consolidated Standards of Reporting Trials diagram, figure 1). We aim to observe the effect of DP-guided individualised PEEP on postoperative pulmonary atelectasis in patients with MO undergoing bariatric surgery. The trial has been designed in accordance with the fundamental principles outlined in the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines for Biomedical Research Involving Human Subjects. The Ethics Committee of the First Affiliated Hospital of Shandong First Medical University has approved this study (YXLL-KY-2023(144)). The investigator will promptly report all the changes in the study (such as revisions to the protocol and/or informed consent form) to the ethics committee.
Consolidated Standards of Reporting Trials diagram. PEEP, positive end-expiratory pressure.
Participants
Patients with MO scheduled for bariatric surgery will be assessed during the routine preoperative visit. Eligible participants will voluntarily provide written informed consent containing the objectives, required follow-up, risks, safety measures and their right to withdraw from participation at any time. The personal information of the participants will be kept strictly confidential. From 1 March 2024 to 30 April 2025, 52 participants with MO scheduled for laparoscopic bariatric surgery will be enrolled in our study.
Inclusion criteria
Age between 18 and 60 years old.
American Society of Anesthesiologists (ASA) classification I–III.
Body mass index (BMI)≥35 kg/m2.14
Intermediate or high risk of PPCs defined by the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score (with an ARISCAT score≥26).15
Signed the informed consent form.
Exclusion criteria
Respiratory infection within 4 weeks, chronic obstructive pulmonary disease or other chronic lung diseases, history of pulmonary and thoracic surgery and deformity of the chest wall or thoracic spine.
Neuromuscular dysfunction.
Serious cardiac, renal, hepatic and haematopoietic diseases.
Contraindications of PEEP (bronchopleural fistula, intracranial hypertension, etc).
Standard procedures
General procedures
In order to ensure a high quality of anaesthesia management and to control confounding factors, several common strategies will be implemented:
All study participants will receive an ultrasound-guided transversus abdominis plane block with 40 mL of 0.5% ropivacaine (left: 20 mL; right: 20 mL) before surgery. Additionally, postoperative analgesia will be provided to maintain the Visual Analogue Scale pain score below 3.
Haemodynamic management will be guided by intraoperative monitoring of circulation, and fluids will be administered according to goal-directed therapy principles.
Antibiotic prophylaxis will be administered to prevent infection, and pharmacological prophylaxis will be used to prevent postoperative nausea and vomiting.
Following the operation, the study participants will be transferred to the post-anaesthesia care unit and administered sugammadex sodium to reverse inotropic relaxation completely.
Extubation will be conducted according to strict extubation indications.
Postoperative physical therapy will include early mobilisation and cough stimulation, as well as deep breathing exercises.
Monitoring
On admission to the operating room, the following parameters will be monitored: ECG, non-invasive blood pressure (BP), pulse oxygen saturation (SpO2), end-tidal carbon dioxide concentration (EtCO2), bispectral index (BIS), invasive BP by radial artery catheterisation and nasopharyngeal temperature. Ventilatory parameters will be monitored by the mechanical ventilator. Postoperative monitoring will include ECG, SpO2 and non-invasive BP at least.
Anaesthesia protocol
Participants will receive routine intravenous rapid intravenous anaesthesia induction based on ideal body weight with midazolam 0.05 mg/kg, etomidate 0.3 mg/kg, rocuronium 0.6 mg/kg and sufentanil 0.5 µg/kg. Following intubation, propofol and remifentanil will be continuously administered to maintain a BIS value of 40–60 and keep heart rate (HR) and BP fluctuations within a 20% range of the baseline. Rocuronium will be administered to maintain muscle relaxation. Any anaesthesia-related complications will be treated according to the most recent clinical guidelines.
Mechanical ventilation
We will connect the ventilator immediately following the intubation. Mechanical ventilation will be conducted in the volume-controlled ventilation mode (VCVM) with tidal volume (VT) of 6–8 mL/kg predicted body weight, fraction of inspiration O2 (FiO2) of 40%, respiratory rate (RR) of 12 breaths per minute, and inspiration-to-exhalation ratio of 1:2. All the parameters can be adjusted as needed to maintain SpO2≥92% and EtCO2 between 35 and 45 mmHg.
Intervention
Driving pressure group (DP group)
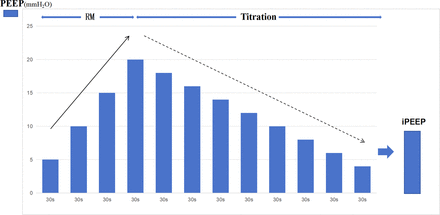
In the DP group, we will conduct individualised PEEP titration at three time points throughout the operation (figure 2): T1—after intubation, T2—after establishment of pneumoperitoneum and T3—after exsufflation of pneumoperitoneum. The recruitment manoeuvre (RM)+PEEP titration mode (figure 3) will be the method to achieve the individualised PEEP: RM to open the collapsed alveoli and PEEP titration to achieve the lowest DP. The anaesthesiologist will have to ensure haemodynamic stability before, during, and after the RM+Titration.
Individualised PEEP titration at three time points. T1—after intubation, T2—after establishment of pneumoperitoneum, and T3—after exsufflation of pneumoperitoneum. iPEEP, individualised PEEP; PEEP, positive end-expiratory pressure; RM, recruitment manoeuvre.
Driving pressure-guided individualised PEEP titration process. iPEEP, individualised PEEP; PEEP, positive end-expiratory pressure; RM, recruitment manoeuvre.
Recruitment manoeuvre
The procedure will be initiated with RM, and the ventilator parameter settings will be as follows: pressure-controlled ventilation mode (PCVM), RR 6 breaths per minute, inspiratory-to-expiratory ratio 1:2, PEEP 5 cmH2O, and inspiratory pressure 20 cmH2O. Then we will increase the PEEP from 5 to 20 cmH2O by 5 cmH2O intervals. Each PEEP level will be maintained for 30 s. The DP will be maintained at 15 cmH2O throughout the process to prevent barotrauma. Figure 3
Titration
Subsequently, PEEP will be titrated in a decremental pattern. In VCVM, PEEP will be decreased from 20 to 4 cmH2O by 2 cmH2O intervals. Each PEEP level will be maintained for 30 s. All other ventilation parameters except PEEP will be the same as those in VCVM described in the Mechanical ventilation section. The DP at each PEEP level will be calculated, and the PEEP corresponding to the lowest DP will be identified as the individualised PEEP (iPEEP) for the participant. If multiple PEEP levels show the same minimum DP, we will select the lowest PEEP. Titration at three time points will be to achieve dynamic individualised PEEP (iPEEP 1, iPEEP 2 and iPEEP 3). Figure 3
Fixed PEEP group (FP group)
In the fixed PEEP group, PEEP will be fixed at 8 cmH2O throughout the mechanical ventilation, and other ventilation parameters will be the same as those in the VCVM described in the Mechanical ventilation section.
Study endpoints
Primary outcome
The primary outcome is the extent of postoperative atelectasis 30 min after extubation measured by modified lung ultrasound score.
Lung ultrasound examination procedure
All ultrasound examinations will be performed by an experienced ultrasound expert using Mindray M9 with a convex probe of 1.3–5 MHz. The probe will initially be placed perpendicular to the ribs to identify the ‘bat sign’, then, it will be placed laterally between the intercostal spaces to avoid interference from the upper and lower ribs and to expose the pleural line well. Scanning will be conducted in six regions per hemithorax (12 quadrants in total): anterior, lateral and posterior fields are identified by the sternum, anterior and posterior axillary lines, with each field being subdivided into superior and inferior regions.16 We will scan the paravertebral regions by turning the participant to the lateral position and will use smaller probes to provide a more precise and comprehensive evaluation of actual atelectasis regions.
The modified lung ultrasound score (incorporates the assessment of small subpleural consolidation based on the original lung ultrasound score (LUS))17 distinguishes four progressive steps of loss of aeration according to the artefacts visualised in a scan (online supplemental table 1):
Supplemental material
The LUS (0–36) will be calculated by adding up the scores for the 12 quadrants, with higher scores indicating more severe aeration loss. We define atelectasis to be significant if any region has a score of≥2.
Secondary outcomes
Lung ultrasound score on postoperative day 1.
The incidence and severity of PPCs within 3 days after surgery (diagnostic criteria are defined by the severity of PPCs).18
Mechanical respiratory parameters include peak airway pressure (Ppeak), airway plateau pressure (Pplat), static lung compliance (Cst), DP, PEEP and mechanical power (MP).
Indicators of the arterial blood gas: PaO2, PaCO2, OI (PaO2/FiO2).
The incidence of hypotension during titration and the dosage of vasoactive drugs.
Adverse events and countermeasures
Due to individual responses during the application of RM and titration, adverse effects such as hypotension may occur in a small proportion of participants.5 All study participants will be administered prophylactic norepinephrine 4 µg to prevent hypotension (defined as mean arterial pressure<65 mmHg) before per RM. If hypotension persists during recruitment or titration, standard vasoactive drug usage methods and fluid goal-directed therapy principles will be used to maintain haemodynamic stability. In the event of the following occurrences, the titration operation will be stopped and an appropriate treatment or rescue measures will be promptly initiated: (1) SpO2<92%, (2) HR>140 beats per minute or<50 beats per minute, (3) mean arterial pressure<60 mmHg or decrease from baseline of 20% and (4) any new-onset arrhythmia appears. We will record all the treatment and rescue measures used and will exclude participants with ≥2 interruptions.
Study timeline
The participants will be followed up preoperatively, intraoperatively and postoperatively (online supplemental table 2). At different stages, data will be collected and documented as follows:
Supplemental material
Preoperative indicators
Age, sex, height, weight, BMI, smoking history, ASA, ARISCAT risk score, BP, HR, temperature, blood routine, biochemical parameters, coagulation function, history of present illness, and history of past illness, etc.
Intraoperative indicators
BP, HR, SpO2, BIS and respiratory parameters (VT, PEEP, Ppeak, Pplat, DP, Cst and MP) will be recorded at three different time points (5 min after each titration).
Blood gas analysis (T0—before anaesthesia induction, T2 and T4—30 minutes after extubation).
Type and volume of fluids; blood loss and transfusion requirements; urine volume and diuresis; dosage of analgesic, sedative, muscle relaxant, vasoactive drugs; duration of surgery and mechanical ventilation time.
Postoperative indicators
Lung ultrasound score, hypoxaemia, incidence and severity of PPCs, etc.
Sample size calculations
Based on our unpublished pre-experimental data, the mean±SD LUS 30 min after extubation was 4.79±1.46 when a fixed PEEP of 8 cmH2O was used. We hypothesised that DP-guided PEEP will lead to a 30% reduction in LUS.19 With α=0.05, 1-β=0.9 and a dropout rate of 10%, the sample size is calculated to be 52.
Randomisation, allocation, blinding and concealment
Eligible participants will be randomly assigned to the DP group and FP group using block randomisation in a ratio of 1:1. Except for the anaesthesiologist, participants, observers, surgeons, researchers evaluating outcomes, statisticians and all other researchers will be blinded to the allocation. After the induction of anaesthesia, the anaesthesiologist will receive a sealed envelope containing the allocation information. Then, the corresponding ventilation strategies will be implemented.
Data collection and management
Data will be collected by independent researchers who are blinded to the allocation. The original data will be recorded in the Case Report Form, and the records must be timely, true, accurate and complete. The Data Monitoring Committee will be responsible for monitoring and evaluating data from clinical trials. Before data analysis, the pattern of missing data will be evaluated. For subjective data, the method of case deletion for missing values will be employed; for objective data, the imputation method of missing values will be employed; for illogical data, the method of setting the null value or filling the average value will be employed. The study data will be entered into a designed Microsoft database and will be kept for 10 years after the end of the study. The original data and results will be submitted to the Scientific Review Board at the end of the study and will be made available to the public after the article is published. Only the investigators in this study will have access to these data.
Statistical analysis
All data will be analysed using SPSS V.27.0. Discrete variables will be presented as frequencies or percentages, and the χ2 test or Fisher’s exact test will be used for intergroup comparisons. For continuous variables, data conforming to the normal distribution will be expressed as mean±SD (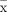 ±s), and between-group differences will be assessed with a t-test or analysis of variance. Data conforming to the non-normal distribution will be expressed as the median and IQR, and between-group differences will be assessed with the Mann-Whitney U test. Covariance analysis and logistic regression analyses will be used to control the influence of confounding factors.
±s), and between-group differences will be assessed with a t-test or analysis of variance. Data conforming to the non-normal distribution will be expressed as the median and IQR, and between-group differences will be assessed with the Mann-Whitney U test. Covariance analysis and logistic regression analyses will be used to control the influence of confounding factors.
Discussion
The combined effect of compression on the dorsal-caudal aspect of the lung and gas absorption in lung units exposed to small airway closures results in a greater risk of perioperative pulmonary atelectasis in obese patients.20 21 Perioperative atelectasis leads to a decreased end-expiratory lung volume, ventilation/perfusion mismatch and reduced lung compliance.22 23 This may contribute to the development of PPCs.24 As a perioperative lung-protective ventilation strategy, individualised PEEP guided by DP deserves further investigation.
In our study, the RM combined with decreasing PEEP titration mode will be adopted. The study has demonstrated that the combination of individualised PEEP and RM achieved better physiological effects.25 The pneumoperitoneum compresses the juxta-diaphragmatic lung regions by increasing intra-abdominal pressure, thus promoting the cephalad displacement of the diaphragm.26 The use of pneumoperitoneum impacts the optimal PEEP level,9 so we will repeat the titration procedure dynamically after the insufflation and exsufflation of pneumoperitoneum. The MP of the two ventilation strategies will be documented in this study. This unifying metric reflects the energy delivered by the ventilator to the respiratory system over time. Broader physical and physiological considerations require an examination of the overall mechanical stress imposed on the lungs by ventilation and the emerging concept of MP.27
Although chest CT is the gold standard for identifying postoperative atelectasis, patients seldom get routine chest radiography or CT examinations following surgery because of the radiation risk and the inconvenience of travelling back and forth to the radiology department. Consequently, asymptomatic atelectasis and other PPCs are often overlooked in the early postoperative period. In recent years, bedside lung ultrasound has been applied in the perioperative period due to its advantages of simplicity, safety, reproducibility and non-radiation. The diagnostic accuracy of LUS for atelectasis is up to 97.2%.28 It is an effective tool for evaluating perioperative pulmonary complications. The scoring system for measuring the extent of atelectasis has been validated in numerous publications.17
DP-guided individualised PEEP has shown superiority not only in non-obese surgical patients10–13 29 but also in obese patients. Yang et al 30 observed that an individualised PEEP-based DP-guided ventilation strategy could reduce intraoperative DP and increase respiratory compliance in patients with obesity undergoing laparoscopic sleeve gastrectomy. However, the effect of DP-guided individualised PEEP on postoperative atelectasis in patients with MO remains unclear. Therefore, this study intends to use lung ultrasound to observe the effect of DP-guided individualised PEEP on atelectasis after bariatric surgery in patients with MO and to provide a reference for clinical application. It is of great significance to reduce the occurrence of atelectasis by optimising the ventilation strategy.
Ethics and dissemination
This study has been approved by the Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (YXLL-KY-2023(144)). The trial results will be published in peer-reviewed journals and at conferences.
Supplemental material
Ethics statements
Patient consent for publication
Ethics approval
This study has been approved by the Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (YXLL-KY-2023(144)).
Acknowledgments
We sincerely thank the staff at the Center for Big Data Research in Health and Medicine and the doctors of the ultrasound department in the First Affiliated Hospital of Shandong First Medical University.
References
Footnotes
Contributors YW and YTS were the principal investigators of this study, designed and refined the study protocol. YTS is the guarantor. YR drafted the protocol and wrote the protocol manuscript. YR, PZ, LC, PL and ZZ assisted in the development and implementation of the study. YW and YTS provided the supervision support. All authors critically reviewed and approved the final manuscript.
Funding This study was funded by the National Natural Science Foundation of China (82270093), Academic Promotion Programme of Shandong First Medical University (2019QL015), Shandong Medical Association Clinical Research Fund—Qilu Special Project (YXH2022DZX02007) and Natural Science Foundation of Shandong Province (ZR2022MH221).
Competing interests The authors declare that the research will be conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.