Article Text
Abstract
Objectives Older people living with HIV (PLWH) globally are experiencing a combination of both communicable and non-communicable disease (NCD) morbidities. Vascular contributions to cognitive impairment and dementia (VCID) can contribute to adverse ageing brain health. This study aimed to measure VCID and HIV-related factors and evaluate their association with cognitive performance.
Design A cross-sectional study.
Setting Five cities in the country of Georgia.
Participants We enrolled PLWH age ≥40 years. Recruitment and data collection were carried out between February and September 2023. We conducted face-to-face interviews and collected data on sociodemographic characteristics, medical history, HIV history, cardiovascular health, mental health, clinical measurements and cognitive performance.
Primary outcome measures We calculated the estimated 10-year cardiovascular risk using the Framingham risk score (FRS). Descriptive analyses were conducted using the frequency distributions of relevant categorical variables and median and IQR for continuous variables. Multivariable linear regression analyses were conducted separately for each cognitive assessment score.
Results A total of 125 PLWH aged ≥40 years were enrolled in the study. The median FRS was 9% (IQR: 4, 15), with 37 (30%) participants having intermediate risk and 17 (14%) with high risk of cardiovascular event. In univariate correlation analysis, FRS was associated with worse cognitive performance. The FRS remained associated with worse performance on the Trails Making Test B and Grooved Pegboard Test using multivariable models. On average, every 1 per cent increase in FRS corresponded to an increase of 1.65 s (95% CI: 0.11, 3.19, p=0.04) for completing the Trails Making Test B and an increase of 1.02 s (95% CI: 0.43, 1.60, p=0.001) for completing the Grooved Pegboard Test.
Conclusions We found a high prevalence of cardiovascular risk and an association between this risk and cognitive performance in our sample. Our findings provide a baseline that can be further investigated in larger-scale studies with longitudinal assessment of cardiovascular risk factors and cognitive performance. Furthermore, it can inform the development of policies and programmes to mitigate adverse effects of VCID on the health of PLWH in Georgia and the Eastern Europe and Central Asia region.
- Aging
- Cardiovascular Disease
- Cognition
- HIV & AIDS
Data availability statement
Data are available upon reasonable request. Original data collected within this study is not publicly available, as it might contain sensitive information. De-identified data can be shared based on a reasonable request by sending an email to mdjibuti@prah.ge.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study used comprehensive screening for cardiovascular risk factors and cognitive performance using standardised and validated clinical and blood-based biomarker approaches, increasing its internal validity.
A cross-sectional design does not allow us to assess the change in cognitive performance over time or the temporality of the association between cardiovascular risk factors and cognitive impairment.
Due to the small sample size, we might not have adequate statistical power to generate precise estimates, thus underestimating the extent of the association between cardiovascular risk factors and cognitive performance.
Clinical measurements for cognitive disorders were not part of this study, limiting our ability to assess the clinical significance of the relationship between cardiovascular risk factors and brain health.
Background
The morbidities associated with ageing among people living with human immunodeficiency virus (HIV) is a relatively new area of research. Studies of HIV and ageing in high-income countries show that vascular risk factors (eg, Type 2 diabetes, obesity, hypertension and hyperlipidaemia) are highly prevalent among virally suppressed people living with HIV (PLWH) and among those who are not virally suppressed.1 In addition, PLWH age against a backdrop of co-infections and behaviours that are associated with high vascular risk, such as cigarette smoking and alcohol misuse.2 Thus, ageing PLWH globally are experiencing a combination of both communicable and non-communicable disease (NCD) burden, a first-ever phenomenon of the 21st century. This issue has not been adequately researched in low- and middle-income countries, particularly in the Eastern Europe and Central Asia (EECA) region.3
Vascular risk is important not only for heart health in middle-aged adults but also for the health of the ageing brain.1 4–6 Vascular contributions to cognitive impairment and dementia (VCID),7–9 including obesity, Type 2 diabetes, hypertension, cerebrovascular disease and stroke, are leading causes of death and contribute to late-onset clinical Alzheimer’s disease (AD).5 6 PLWH experience VCID,1 10 which might be exacerbated by certain antiretroviral therapies (ART) that may lead to obesity and hyperlipidaemias.11–13 Lifestyle and behavioural factors common among some PLWH such as smoking,14 substance use,15 poor dietary quality16 17 and lack of physical activity18 comprise other aspects of VCID that are associated with adverse ageing brain health.19
There is a gap in knowledge about VCID among PLWH in the EECA region, including the country of Georgia.20 Although the HIV care continuum in Georgia has substantial gaps in earlier stages of the care cascade, once PLWH enrol in ART, viral load suppression is achieved in 92% of PLWH.21 Thus, more virally suppressed PLWH in Georgia live to older ages, consistent with global trends,22 and cardiovascular and ageing brain conditions are becoming more prevalent. However, to our knowledge, the intersection of VCID with the HIV care continuum has never been assessed in Georgia and EECA, and the prevalence and types of ageing-related cardiovascular comorbidities among PLWH are unknown.
To mitigate adverse effects of VCID on the health of PLWH, there is a need to identify the prevalence of VCID and other ageing-related risk factors in PLWH and characterise relationships between NCDs and brain health. We conducted a cross-sectional study in Georgia to measure VCID and HIV-related factors and evaluate their association with cognitive performance to improve understanding of the surging ‘HIV+NCD’ care continuum and its relation to brain health. This was the first study among ageing PLWH in Georgia and the EECA region that used comprehensive screening for cardiovascular risk factors and cognitive performance using standardised and validated clinical and blood-based biomarker approaches. Our hypothesis was that the prevalence of VCID among PLWH would be higher than that observed in high-income country cohorts and that VCID would be related to poorer cognitive performance. The results of this study will inform the development of innovative interventions to reduce VCID and subsequent adverse brain health outcomes among PLWH in Georgia, as well as future prospective observational studies designed for longitudinal assessment of ageing processes among PLWH.
Methods
Study design, sample and recruitment
We conducted a cross-sectional study among older PLWH in the country of Georgia. Study participants were recruited by community-based organisations working with PLWH using a convenience sampling method. Inclusion criteria were: (1) diagnosed HIV, (2) age ≥40 years and (3) proficiency in the Georgian language. Recruitment and data collection were carried out between February and September 2023 in five major cities of Georgia: Tbilisi (capital city), Gori, Zugdidi, Kutaisi and Batumi.
Data collection
We conducted face-to-face interviews using a paper-based questionnaire and collected data on sociodemographic characteristics, medical history, HIV history, cardiovascular health, mental health and cognitive function. Additionally, we performed clinical measurements, including laboratory blood tests and assessment of physical parameters. Data were collected by a trained interviewer (EI) who had undergone training in data collection standards and techniques with a particular emphasis on cognitive assessments. Before data collection, each participant was provided with detailed information on the data collection procedures, study goals and potential risks of participation. Each participant signed the informed consent form.
Cognitive and mental health assessments
Cognitive performance was measured with the following cognitive assessment tools: (1) the Montreal Cognitive Assessment (MoCA), widely used to detect mild cognitive impairment;23 24 (2) Trail-Making Tests A and B (TMT A and TMT B), assessing executive functions, visual memory, speed of processing, etc;25 (3) letter fluency and semantic fluency for the evaluation of verbal functioning;26 (4) Standard Stroop Tests to assess cognitive flexibility, resistance to outside interference, creativity27 and (5) Grooved Pegboard Test (GPT) to evaluate coordination and motor functions.28 In TMT A, TMT B and GPT, results were measured in seconds to task completion, thus a higher score (time) indicated worse cognitive performance. In the rest of the assessments, higher scores indicated better cognitive performance.
To assess mental health, we used the Georgian versions of widely used instruments: General Anxiety Disorder-7 (GAD-7) to assess anxiety symptoms,29 and Beck’s Depression Inventory (BDI) to evaluate the depression symptoms.30 Participants received clear and concise instructions to complete this survey section independently. Thus, unless participants specifically requested assistance, these assessments were self-administered. We also collected data on substance and alcohol use disorders using standardised instruments—the Drug Use Disorder Identification Test (DUDIT)31 and the Alcohol Use Disorder Identification Test (AUDIT).32
Clinical assessments
A fasting blood sample was taken for each participant, and the following tests were performed: (1) complete blood count (CBC), (2) lipid panel, (3) blood glucose, (4) haemoglobin A1C (HbA1c) and (5) high sensitive C reactive protein (hsCRP). We also measured body weight (kilograms, kg), height (metres, m), heart rate (beats/minute), oxygen saturation via oximetry (%) and systolic and diastolic blood pressures (mm Hg). Body mass index (BMI) was calculated as kg/m2 based on clinically measured body weight and height. All measurements were taken once before the cognitive assessments. Blood pressure was also measured at the end of the interview.
Self-reported assessments
Self-reported data include the number of years on ART and ART interruption, family history of dementia and personal history of the following conditions: hepatitis C virus (HCV) infection, hepatitis B (HBV) infection, COVID-19, tuberculosis, syphilis, stroke and traumatic brain injury.
Variable definitions
We calculated 10 year cardiovascular risk using the Framingham risk score (FRS) that includes the following variables: sex, age, current smoking status, systolic blood pressure, medication use for hypertension, diabetes mellitus, total cholesterol and high-density lipoprotein (HDL) cholesterol.33 We categorised FRS into low (0–10%), medium (10–20%) and high risk (≥20%). The presence of diabetes was defined as HbA1c ≥6.5% or a self-reported diagnosis of diabetes. HbA1c between 5.7% and 6.4% was classified as ‘high-risk of diabetes’. Total blood cholesterol in the range of 200–239 mg/dL was classified as ‘above desirable’ and ≥240 mg/dL was classified as ‘high’. HDL was classified as normal (≥40 mg/dL) or low (≤39 mg/dL). Underweight was defined as BMI <18.5 kg/m2, normal or healthy as BMI=18.5–24.9 kg/m2, overweight as BMI=25.0–29.9 kg/m2 and obesity as BMI ≥30 kg/m2.
Drug dependency was defined as a DUDIT score of ≥2 for females and ≥6 for males, or a respondent being enrolled in opioid agonist therapy (OAT). BDI score was categorised into no depression (0–16) and borderline or clinical depression (≥17). GAD7 score was dichotomised into minimal or mild anxiety (0–9) and moderate or severe anxiety (10–21).
Statistical analysis
Descriptive analysis was conducted using the frequency distributions of relevant categorical variables and median and IQR for continuous variables. Continuous variables were categorised only in the descriptive analysis and tables but were treated as continuous in linear regression analyses. Due to the lack of standardised adjustments for cognitive assessment results based on local context, all cognitive results were analysed as raw scores. Bivariate analysis was conducted to assess crude associations between demographic and health-related variables and cognitive outcomes. The main exposure of interest was cardiovascular risk using the FRS.33 First, we descriptively explored the differences in cognitive performance by FRS categorised into low, intermediate and high risk. Pearson correlation coefficients were calculated to assess the association between continuous FRS and cognitive assessment scores. To select covariates for inclusion in the multivariable analyses, we set a p value <0.10 as the level of statistical significance in the bivariate analysis. Variables evaluated as potential covariates included: sex, age, level of education, whether a participant had enough income for basic needs, smoking status (current, past, never smoker), drug-dependency, BMI, receiving social aid, marital status, number of people in the household, AUDIT score, BDI score, GAD7 score, number of years on ART, family history of dementia and history of the following conditions: HCV infection, HBV infection, COVID-19, tuberculosis, syphilis, stroke, traumatic brain injury and ART interruption. Covariates were included in all multivariable analyses if they were statistically significantly associated with at least three cognitive outcomes. The same set of covariates was included in all final multivariable models. Multivariable linear regression analyses were conducted separately for each cognitive assessment score. After exploring the FRS as an exposure of interest, we also ran the linear regression models with individual risk factors as exposures of interest: total cholesterol, HDL, diabetes and smoking, additionally adjusted for every other component of the FRS. In all multivariable analyses, the significance level was set at 95% and p value <0.05 was considered statistically significant. Missing data was handled using complete-case analysis. All statistical analyses were conducted using R software (V.4.3.2).34
Ethics
The study protocol and data collection instruments were approved by the Institutional Review Board at the Georgian National Centre for Disease Control and Public Health (IRB00002150) before data collection started (Approval number IRB # 2022–076). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Patient and public involvement
Representatives of patient organisations and civil society organisations were involved in research design, implementation planning and recruitment processes.
Results
Demographic, behavioural and clinical factors
A total of 125 PLWH aged ≥40 were enrolled in the study. Of them, 47 (38%) were females, and the median age was 49 years (IQR: 44–54) (table 1). Approximately half (n=61, 49%) had <12 years of education, and 37 (30%) received social aid. More than half of the participants were either overweight (n=47, 38%) or obese (n=16, 13%). Approximately two-thirds were current smokers (n=80, 65%), and an additional 14 (11%) were past smokers.
Descriptive statistics of sociodemographic and clinical factors among older people living with HIV, Georgia, 2023 (n=125)
FRS was calculated for 122 participants. The prevalence of cardiovascular risk factors was high overall in our study population. A median FRS was 9% (IQR: 4, 15). After categorising the FRS, 37 (30%) participants had intermediate risk, and 17 (14%) had a high risk of cardiovascular event. Six participants (4.8%) had diabetes, including three with HbA1c ≥6.5% and three with self-reported diagnosis of diabetes. Additionally, 14% (n=18) of participants were at high risk of diabetes (HbA1c between 5.7% and 6.4%). Total cholesterol was above desirable or high in 19.5% (n=24) participants, and HDL was lower than the optimal in 33% (n=40).
Association between cardiovascular risk factors and cognitive performance
There was slight variability in MoCA score, with a mean score of 19 in people with high FRS and a mean score of 21 in people with low or intermediate risk (table 2). Regarding TMT A and TMT B, people with high FRS required more time to completion than those with intermediate or low FRS. Similarly, people with high FRS scored lower on letter fluency and semantic fluency tests. Regarding the GPT, persons with high FRS took on average 114 s (SD=27) to complete the test, compared with 90 (SD=21) seconds in persons with intermediate FRS and 88 (SD=28) among people with low FRS.
Difference in cognitive assessment scores by Framingham risk score categories among older people living with HIV, Georgia, 2023 (n=125)
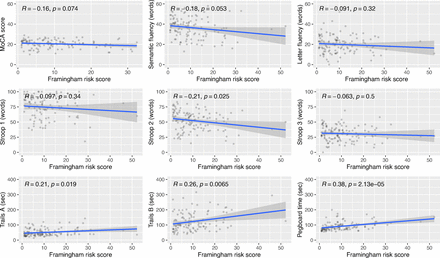
In correlation analyses, the FRS was statistically significantly associated with several cognitive assessment results: TMT A (r=0.21, p=0.019), TMT B (r=0.26, p=0.007), semantic fluency (r=−0.09, p=0.032), Stroop test 1 (r=−0.21 p=0.022), Stroop test 2 (r=−0.21, p=0.025) and GPT (r=0.38, p<0.001). Among all assessments, a higher FRS was predictive of worse performance (figure 1).
Correlation between Framingham risk score and cognitive assessment results among older people living with HIV, Georgia, 2023 (n=122). Pearson’s correlation test was used to generate the p values. Note: Trails A, Trails B and the pegboard test are measured in time to task completion, thus a higher score (time) indicates worse cognitive performance.
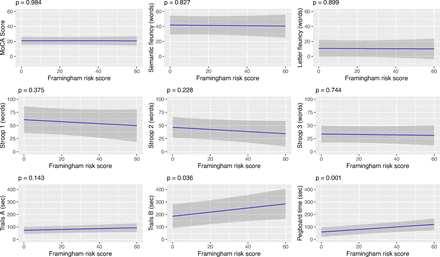
In the primary multivariable analyses, linear regression models were adjusted for BMI, years of education, drug dependency, having enough income for basic needs, history of HCV infection, history of TB, history of stroke, history of COVID-19 and history of ever interrupting ART. The Framingham risk score remained associated with the Trails Making Test B and Grooved Pegboard Test. On average, every one per cent increase in FRS corresponded to an increase of 1.65 s (95% CI: 0.11, 3.19) to complete the TMT B and to an increase of 1.02 s (95% CI: 0.43, 1.60) needed to complete the GPT (table 3, figure 2). Multivariable analyses evaluating individual cardiovascular risk factors were additionally adjusted for age, sex and other non-overlapping components of FRS. Total cholesterol was associated with worse performance in the letter fluency test. Diabetes was statistically significantly associated with better performance in letter fluency and semantic fluency tests. Current smoking was not statistically significantly associated with any of the cognitive assessment results (data not shown).
Association between Framingham risk score and cognitive assessment results among older people living with HIV, Georgia, 2023 (n=122). Results from multiple linear regression model adjusted for BMI, years of education, drug dependency, having enough income for basic needs, history of HCV infection, history of tuberculosis, history of stroke, history of COVID-19 and history of ever interrupting ART. Note: Trails A, Trails B and pegboard test are measured in time to task completion, thus a higher score (time in seconds) indicates worse cognitive performance. ART, antiretroviral therapies; BMI, body mass index
Multivariable analyses of association between cardiovascular risk factors and the cognitive assessment results, older people living with HIV, Georgia, 2023 (n=122)
Discussion
To our knowledge, this was the first study assessing the prevalence of VCID and their relationships with cognitive performance among older PLWH in the country of Georgia. We found a high prevalence of VCID similar to high-income settings,35 and an association between the FRS and cognitive performance. While the causality and directionality of this association cannot be determined from this cross-sectional study, our findings provide a baseline that can be further investigated in larger-scale studies with longitudinal assessment of VCID and cognitive performance.
We found that the FRS was associated with lower cognitive performance among older PLWH across multiple domains and retained for executive function measured via TMT B and motor function measured using the GPT with multivariate adjustment, which aligns with previous studies.36 37 This was not observed with the evaluation of individual vascular risk factors as exposures of interest. This might suggest that a combination of risk factors contributes to cognitive impairment and, therefore, it is beneficial to use the FRS or other similar tools as primary exposures.
That we only found an association of cardiovascular risk with some domains of cognition is consistent with the literature. It has been hypothesised that cardiovascular changes precede cognitive impairment across all or several domains,38 particularly for episodic memory, working memory and perceptual speed,39 40 and that an exposure, such as obesity, precedes poorer cognitive performance.41 Our cross-sectional study design does not allow determination of temporality. Other longitudinal studies found that the FRS is predictive not only for cardiovascular risk but also for cognitive decline.40 This further emphasises the importance of longitudinal studies.
Our findings in this older sample of PLWH >=40 years may not be related to HIV alone but to comorbid vascular, inflammatory and other ageing-related brain and peripheral events. While our study was not designed to compare PLWH and people without HIV, existing evidence suggests that PLWH might be more affected by this dual burden due to the additive or synergistic effect of HIV infection and vascular risk factors on brain health.42 Mechanisms for this association are likely multifactorial. Previous studies found a significant effect of cardiovascular risk factors on brain structure among PLWH through changes in cerebral small vessels and microstructure of white matter.39 43–45 One of the potential mechanisms is high prevalence of hypoechoic plaques found in PLWH with CD4 <200/mm3 suggestive of enhanced continued inflammation.46 Furthermore, inflammatory markers that contribute to cognitive impairment are common with both cardiovascular risk factors and HIV.47–49
Unexpected findings of our study were the following. First, there was a positive association between the presence of diabetes and better performance in semantic fluency and letter fluency tests. Previous studies mainly suggested that diabetes is associated with decreased cognitive performance across several cognitive domains, including as measured using semantic and letter fluency assessments.50 51 This discrepancy may be explained by potential misclassification due to a self-reported diagnosis of diabetes, while our definition of diabetes relied on both self-report and lab results of HbA1c. Another reason for this discrepant result could be a small number of people with diabetes in our sample (n=6), even with the combined variable. When we repeated the analysis using only HbA1c results, we did not see any statistically significant association with the cognitive assessment results (data not shown). Second, the average score on the MoCA was low, suggesting that the majority of our sample had cognitive impairment or dementia. Clearly, this was not the case when compared with neuropsychological performance assessment results, which indicated that performance was not generally impaired. Despite the MoCA being available in several languages with slightly different forms, more information is needed related to the interpretation of the scores. Previous research in Georgia also found lower than expected scores on MoCA and recommended a lower cut-off for determining cognitive impairment.23
Our study has several limitations. First, as aforementioned, a cross-sectional design does not allow us to assess the change in cognitive performance over time or the temporality of the association between cardiovascular risk factors and cognitive impairment. Second, our study did not involve a comparison group of people without HIV, limiting our ability to explore the interplay between ageing, HIV infection and cardiovascular risk factors more thoroughly. Third, due to the small sample size, we might not have adequate statistical power to generate precise estimates, thus underestimating the extent of the association between cardiovascular risk factors and cognitive performance. Fourth, we did not clinically measure cognitive disorders, which would require a diagnostic evaluation by clinicians and additional clinical investigations, such as neuroimaging. Therefore, we cannot assess the clinical significance of the relationship between cardiovascular risk factors and brain health. Fifth, we did not have an opportunity to conduct lab tests for viral load and CD4 cell count to explore their impact on cognitive and cardiovascular findings in our study. Although we collected self-reported information on the most recent results of those tests, due to the large proportion of missingness and unreliability of self-reported results, we did not use those variables in the analysis. Lastly, convenience sampling prevents us from generalising our findings to all PLWH in Georgia.
In conclusion, in this cross-sectional study, we found evidence of an association between cardiovascular risk factors and cognitive performance among older PLWH in Georgia. Despite a small sample size and inability to assess the temporality of this association, our study was an important first step in Georgia to generate evidence-based information on the interplay between ageing, HIV, brain health and cardiovascular risk factors. Our findings will contribute to improving holistic care for older PLWH. In addition, our study provides a baseline estimate of the prevalence of cardiovascular risk factors and cognitive performance among older PLWH that can serve as groundwork for larger-scale studies that can assess this association longitudinally.
Data availability statement
Data are available upon reasonable request. Original data collected within this study is not publicly available, as it might contain sensitive information. De-identified data can be shared based on a reasonable request by sending an email to mdjibuti@prah.ge.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and the study protocol and data collection instruments were approved by the Institutional Review Board at the Georgian National Center for Disease Control and Public Health (IRB00002150) before data collection started (Approval number IRB # 2022-076). The study was conducted in accordance with the principles of Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors would like to thank the study participants for their time and efforts; to the following community-based organisations for facilitating the recruitment of the study participants: 'Real People Real Vision', 'HIV-positive Women - We Exist', 'Step to the Future', 'HIV/AIDS Patients Support Foundation'; and to the staff of the laboratory Mrcheveli and its regional branches for supporting the study and providing space for study procedures and high-quality laboratory services.
References
Footnotes
Contributors The study was conceptualised by DRG, MD and JD. The detailed protocol and methodology were developed by DB, EI, DRG and MD. AK provided cognitive assessment tools, trained the interviewers and contributed to the methodology for conducting cognitive assessments. Interviews and data entry were conducted by EI and DB. Statistical analysis was conducted by DB, with guidance from DRG, MD and JD. The manuscript was drafted by DB. All authors reviewed the manuscript, provided feedback and approved the final draft. DB is the guarantor of this manuscript. AI tool (Google Gemini) was used in the process of debugging the code to generate the figures.
Funding The research was supported by the Fogarty International Center and the Office of AIDS Research of the National Institutes of Health under Award Number D43TW011532 and MACS/WIHS Combined Cohort Study, Brooklyn Clinical Research Site, National Institutes of Health U01-HL146202.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.