Article Text
Abstract
Introduction Many chemotherapy agents used to treat advanced cancer are inherently mucotoxic, causing breakdown of the gastrointestinal mucosa (gastrointestinal mucositis (GI-M)) and lead to a constellation of secondary complications including diarrhoea, malnutrition, anorexia, pain, fatigue and sleep disturbances. These symptoms are usually managed individually, leading to polypharmacy and its associated risks. The endocannabinoid system regulates numerous biological and behavioural processes associated with chemotherapy side effects, suggesting its modulation could control these symptoms. Therefore, the CANnabinoids in CANcer (CANCAN) therapy trial is a phase II, randomised, double-blind, placebo-controlled trial that aims to determine the efficacy of medicinal cannabis in minimising GI-M and its associated symptom burden.
Methods and analysis The CANCAN trial is being conducted at four Australian sites: the Royal Adelaide Hospital, the Queen Elizabeth Hospital, Flinders Medical Centre and the Lyell McEwin Hospital. Adults (n=176) diagnosed with a solid tumour or a haematological cancer scheduled to receive mucotoxic chemotherapy will be eligible. Participants will be randomised 1:1 to receive either the investigational product (IP) or placebo, both delivered as sublingual wafers. The active IP contains cannabidiol (300 mg/day) and Δ9-tetrahydrocannabinol (5–20 mg/day, titrated by the participant). The primary outcome is GI-M burden, determined by the Mucositis Daily Questionnaire. Secondary and tertiary outcomes include overall symptom burden (Edmonton Symptom Assessment Scale), anorexia (Average Functional Assessment of Anorexia/Cachexia Therapy), depression/anxiety (Hospital Anxiety and Depression Scale), financial toxicity (Functional Assessment of Chronic Illness Therapy COmprehensive Score for financial Toxicity), quality of life (European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire), incidence of chemotherapy dose reductions/modifications, cumulative dose of chemotherapy administered, incidence/length of hospitalisation, the use of supportive care, and the cost-benefit of the IP. The CANCAN trial prioritises patient experiences by focusing on patient-reported outcome measures and administering medicinal cannabis during active treatment to prevent symptoms that occur secondary to mucositis.
Ethics and dissemination The protocol has been approved by Central Adelaide Local Health Network Human Research Ethics Committee (2022HRE00037). All participants will be required to provide written or digitally authorised informed consent. Trial results will be disseminated in peer-reviewed journals, and at scientific conferences.
Trial registration number ACTRN12622000419763.
- CHEMOTHERAPY
- ONCOLOGY
- HAEMATOLOGY
- Randomised Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Prioritises electronic patient-reported outcome measures to capture a broad range of symptoms while reducing participant burden through enabling efficient completion.
Evaluates the synergistic effects of prophylactic cannabidiol and Δ9-tetrahydrocannabinol (THC) through a personalised dosing strategy, allowing participants to self-titrate to their maximum tolerated THC dose.
Includes a pharmacokinetics sub-study.
Tiered enrichment strategy that enables exclusion of participants who do not develop gastrointestinal mucositis, as well as early intervention to investigational product.
High risk of drop-out/non-adherence due to daily questionnaires, long study duration and driving restrictions during active trial period.
Introduction
Cancer therapy, including chemotherapy, causes a range of debilitating side effects that impact the quality of life (QoL) of people living with or beyond cancer. Currently, these side effects are managed reactively and in isolation.1 2 This approach is flawed for two major reasons: (1) it fails to acknowledge data that show early intervention with supportive care services is more effective and improves QoL3 and (2) it increases the risk of polypharmacy (prescription of ≥5 medications) and its associated risk including hospitalisation and death.4–7 Identifying streamlined strategies to proactively control or prevent multiple symptoms is therefore an area of intense investigation in the field of supportive oncology.
A strong body of evidence shows that damage to the gastrointestinal microenvironment caused by chemotherapy (ie, gastrointestinal mucositis (GI-M)) is a major catalyst for secondary complications1 8 9 (please see Wardill et al., 2023 for a comprehensive overview of this concept). GI-M is initiated by direct cytotoxicity caused by chemotherapy and other cytotoxic agents that are unable to discriminate between the highly proliferative cells of the tumour and those that line the gastrointestinal tract.9 This damage then initiates secondary events that perpetuate mucosal damage, including the production of reactive oxygen species, pattern recognition receptor activation and the production of proinflammatory cytokines.9 The extent and duration of this secondary inflammation ultimately dictate the severity of mucosal injury, which presents as confluent, ulcerative lesions throughout the entire gastrointestinal tract, and associated symptoms such as diarrhoea, bleeding and pain.9 In addition to proinflammatory mechanisms, damage to the mucosa also disrupts the delicate ecosystem of micro-organisms that reside in the gut (the gut microbiota), leading to dysbiosis.10 Dysbiosis, defined as a (typically pathogenic) change in the composition of the gut microbiota, increases the production of microbially produced danger signals (eg, endotoxin/lipopolysaccharide) which further triggers proinflammatory responses via the activation of pattern recognition receptors, including toll-like receptor 4.11 This, combined with a loss in mucoprotective metabolites such as short chain fatty acids, further contributes to mucosal barrier injury and systemic inflammatory responses.11
These secondary events related to GI-M lead to a constellation of symptoms, including infection, malnutrition/weight loss, anorexia, sleep disturbances, cognitive dysfunction and fatigue.9 While these symptoms are not universally recognised or defined as a ‘symptom cluster’, the mechanisms of this symptom profile provides a compelling case that GI-M is a catalyst for number of inter-related symptoms, suggesting that targeting GI-M may deliver widespread and meaningful benefits to patients by addressing numerous symptoms. However, there are currently no effective approved interventions to prevent gastrointestinal symptom clusters in people undergoing cytotoxic cancer therapy.
Medicinal cannabis has gained recent attention for its ability to modulate various organ systems and biological and behavioural processes. Given this, it offers a potential therapeutic avenue to mitigate the symptoms and side effects of chemotherapy. Cannabis extracts are complex botanical mixtures comprised primarily of the exogenous cannabinoids, Δ9-tetrahydrocannabinol (Δ9-THC or THC) and cannabidiol (CBD). These cannabinoids exert their effects primarily through the endocannabinoid system (ECS), a widespread network composed of receptors, ligands, transporters and enzymes. The ECS is a sophisticated and complex signalling network that spans multiple organ systems, including the nervous, cardiovascular, endocrine and immune systems.12–15
There is strong supporting evidence for both the direct and indirect roles of the ECS and exogenous compounds that interact with this system in modulating gut functions in health and disease.16 Regarding GI-M, CBD is a promising candidate to reduce severity due to its anti-inflammatory properties, ability to suppress cytokine release and its role in minimising inflammatory injury to the gut in colitis.17 In the context of cancer therapy, CBD (300 mg/day) has been shown to decrease graft-versus-host disease in phase II open-label testing, with a reduction in mucosal damage.18 In addition to the anti-inflammatory benefits of CBD, THC has demonstrated varying levels of antiemetic,19 20 analgesic, psychosocial21 and appetite-improving properties22–24 in people with cancer.22 25 Furthermore, emerging evidence suggests that combining CBD and THC provides synergistic therapeutic benefits, enhanced effectiveness and fewer side effects compared with their isolated extracts.26–28 A phase II, randomised, double-blind, placebo-controlled trial found that the addition of combined THC (2.5–30 mg) and CBD (2.5–30 mg) alongside standard antiemetics significantly improved nausea and vomiting. Although there were increased side effects with cannabis, none were severe and most participants preferred cannabis to placebo.19
Given the profound impact on QoL that gastrointestinal symptom clusters cause to people undergoing cytotoxic cancer therapy, an effective intervention is needed. Medicinal cannabis is a promising supportive care approach that may provide meaningful and holistic benefits during cancer therapy. Therefore, the CANCAN trial will investigate the efficacy of medicinal cannabis in controlling GI-M and the constellation of impactful symptoms in people with advanced cancer. The effects of cannabis will be compared with placebo alongside standard care to elucidate its therapeutic potential. With CBD and THC administered concurrently, we anticipate synergistic benefits, with mechanistic prevention of mucotoxicity and well-being benefits that will minimise gastrointestinal distress symptoms and improve QoL.
Hypothesis
Early intervention with CBD/THC will minimise the intensity and duration of GI-M, mitigating secondary physical and psychosocial symptoms, thus supporting adherence to anticancer therapy, and ameliorating the economic burden of chemotherapy side effects.
Methods and analysis
Study objectives
Determine the efficacy of early intervention with the interventional product (IP) in mitigating GI-M caused by chemotherapy.
Determine the flow-on effect of mitigating GI-M on secondary symptoms and QoL.
Determine impact of the IP on incidence of dose reductions or treatment delays.
Quantify economic benefit of early intervention with the IP in reducing hospitalisation, supportive care utilisation and lost opportunity to the individual.
Trial design
The CANCAN trial is a phase II, double-blinded, randomised, placebo-controlled trial with two parallel groups, and a 1:1 allocation ratio. The trial will be recruiting participants from a minimum of three tertiary centres across the State of South Australia (SA), Australia.
Intervention and placebo product
Eligible participants (table 1) will be randomised 1:1, receiving the IP or placebo as a sublingual wafer over the course of three cycles of chemotherapy. The active IP will include CBD (300 mg/day) and THC (5–20 mg/day). CBD and THC will be packaged separately, and as such, will be administered separately to ensure THC dose titrations can be easily achieved. CBD will be administered as a standard 300 mg/day dose (six sublingual wafers, three twice daily) without opportunity for titration or modulation unless in the case of relevant adverse event (AE). THC will be administered at a starting dose 5 mg/day, increasing in 5 mg increments approximately two times per week until the maximum tolerated dose is achieved (note: the maximum dose for participants is 20 mg/day). Packaging will be identical between placebo and medicinal cannabis, manufactured by iX Biopharma. Although there is wide variation in THC and CBD dosing across studies, our dosing strategy was based on the best available evidence.29–32 We selected a dose of 300 mg/day of CBD because several studies have demonstrated benefits at this dosage for a wide range of indications, including graft-versus-host disease,18 chemotherapy-induced peripheral neuropathy33 and general cancer symptom burden.34 The dosing and self-titration of THC follow recent recommendations29–31 and the IP will be dosed similarly to Grimison et al.19 Notably, while the CBD dose was relatively low, the authors19 32 specified that CBD was included solely to mitigate the psychoactive effects of THC, which differs from our objective of ameliorating GI-M.
CANnabinoids in CANcer therapy trial inclusion and exclusion criteria
Randomisation, allocation concealment and double-blind conditions
Randomisation schedules have been developed by our trial statistician for each site centrally via an electronic platform using random number tables. These will be stratified for site, cancer type and chemotherapy type to ensure even distribution of participants across both arms. Solid tumour participants with a Mucositis Daily Questionnaire (MDQ) score of ≥1 and all haematological cancer participants scheduled to undergo haematopoietic stem cell transplantation will be randomised in a 1:1 ratio to intervention or placebo after their first dose of chemotherapy (C1) and at the time of enrolment (T0), respectively. This enables the participant cohort to be enriched, that is, increasing the rate of GI-M, while also enabling early intervention with the IP. The randomisation schedule will be used by the hospital pharmacy staff to allocate the IP/placebo to participants. All staff and investigators involved in the trial will be blinded. Participants’ data and notes will be referred to using their clinical trial ID number. The code will only be broken in cases of emergency as stipulated in the Data and Safety Monitoring Board Charter.
Recruitment and consent
Potentially eligible participants will be identified at multidisciplinary team meetings and during clinics at each recruitment site and the treating clinician will discuss the clinical trial with the participant at their next consult/appointment. Participants will provide consent in either written or electronic form, with an eConsent module provided by Consentic (please see online supplemental files 1 and 2).
Supplementary video
Supplemental material
Discontinuation/modification and adherence
A participant may be discontinued from the study medication at any time if it is not in their best interest to continue. If a participant is withdrawn from treatment due to an AE, the participant will be followed and treated until the abnormal parameter or symptom has resolved or stabilised. All participants are free to withdraw from participation at any time, for any reason, specified or unspecified, and without prejudice. Adherence will be monitored via the daily questionnaires, with participants required to document whether they took the study medication (Y/N), the dose and the time of consumption. In addition, all dosage containers (irrespective of usage status) will be returned to the pharmacy on the day of each chemotherapy infusion to be logged to account for all doses over the period between each chemotherapy infusion.
CBD has been associated with liver toxicity,35 and it is recommended to closely monitor renal function.36 As such, at each chemotherapy visit, the most recent Complete Blood Picture (CBP)/Multiple Biochemistry Analysis (MBA20) results must be reviewed to identify any indicators of liver toxicity or renal dysfunction.
MBA20 results that require dose modifications to CBD include:
International normalised ratio (INR): >1.5
Alanine aminotransferase (ALT): >5× upper limit of normal (ULN)
Aspartate aminotransferase (AST): >5× ULN
Bilirubin: >3× ULN
Creatinine: >5× ULN
Estimated glomerular filtration rate (eGFR): <30 mL/min
Course of action: CBD to be stopped for seven days. Liver function tests to be repeated and mode of action to be taken as per Stopping Criteria below (table 2).
Investigational product dose modification and stopping criteria
Supplemental material
THC may cause unwanted sedation or agitation/anxiety. This is to be assessed at each clinic visit or home nursing visit using the Richmond Agitation and Sedation Scale (RASS). RASS scores and corresponding actions are shown in online supplemental table 1.
Retention plan
We have worked with our consumer representative to develop an appropriate narrative to ensure participants understand the benefits of participating in the trial, beyond the direct benefits to themselves that they may experience from the intervention under investigation.
This will be combined with a collection of ‘frequently asked questions’ regarding clinical trial involvement.
Participants will be assessed in face-to-face settings twice each cycle, and clinical trial nurses will be appropriately trained in how to ensure participants remain engaged about their participation.
Concomitant care and rescue medication
There will be no rescue medication that is mandated or prevented (bar any medicinal cannabis products). Symptoms can be managed by the individual and treating clinician as per routine standard of care, with the exception of any medicinal cannabis products. These will be monitored by the participant and logged in the digital platform. Clinical records will also be accessed to record all supportive care provided by the participants’ clinical care team.
Primary outcome
The primary outcome will be GI-M burden, determined by the area under the curve (AUC) of the MDQ score/chemotherapy cycle. GI-M burden will be measured daily through the active trial period. Participants will be part of the trial for a maximum of three full cycles of chemotherapy.
Secondary outcomes
GI-M
Number of days with MDQ ≥1
Maximum MDQ/cycle
Average MDQ/cycle
Plasma citrulline (biomarker of GI-M) assessed weekly through treatment
AUC for hypocitrullinaemia (<10 µM)
Body weight and nutrition
Average body weight change/cycle
Body weight change T1 and T2 follow-up
Average Functional Assessment of Anorexia/Cachexia Therapy (FAACT) score/cycle
FAACT score at T1 and T2 follow-up
Symptom burden
Average Edmonton Symptom Assessment Scale (ESAS-r-CS) score/cycle
ESAS-r-CS score T1 and T2 follow-up
Depression, anxiety and QoL
Hospital Anxiety and Depression (HADS) score at T1 and T2 follow-up
EORTC QLQ-C30 score at T1 and T2 follow-up
Supportive care and hospitalisation
Use of rescue medication (participant reported and clinically provided)
Incidence of hospitalisation and/or emergency department presentations
Duration of hospitalisation (days)
Chemotherapy adherence and survival outcomes
Incidence of treatment breaks, delays, or dose reductions
Cumulative dose of chemotherapy
% of intended dose of chemotherapy achieved
Response to treatment defined by Response Evaluation Criteria in Solid Tumors criteria
Progression-free survival
Overall survival
Adverse events
Incidence of Grade 3+ AEs (define by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) (V.5.0))
Incidence of IP discontinuation
Incidence of dose de-escalations (of IP) due to AEs
Economic impact
Financial toxicity/cost analysis (FACIT-COST)
Cost-benefit analysis
Tertiary/exploratory endpoints
Faecal microbiota composition
Peripheral cytokine concentrations
Population-based pharmacokinetic–pharmacodynamic model
Participant timeline
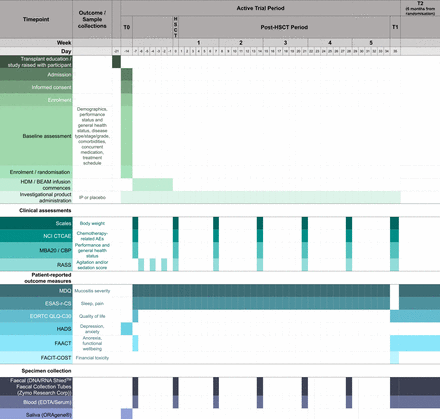
Participants will be identified by their treating clinician, screened and enrolled if eligible by clinical trial and/or members of the investigating team. The intervention will be administered to people with solid tumour and haematological cancers using different approaches that reflect the differences in treatment protocols. All other components of the trial will remain the same for both participating cohorts. The trial will begin recruitment in Q1, 2025 and be open until 176 participants (randomised 1:1) have completed the trial. Study assessments related to trial outcomes will be performed longitudinally across the course of four chemotherapy cycles (three full cycles of study medication). These will be administered at varying frequencies, with key assessments (body weight, AEs, general health status, agitation/sedation score) performed at chemotherapy infusion appointments at the beginning of each cycle. Some assessments will be performed when the participant is not in the hospital. These include the MDQ and other patient-reported outcome measures (PROMs) (completed by participant via PersonifyCare app) and day 7 assessments (mid cycle) which are administered by nursing staff who will visit participants in their home. Support for participants titrating their IP dose will also occur outside the hospital, facilitated by phone calls by nursing staff. A more comprehensive overview of the study assessments and participant timelines is presented in figures 1–3 and online supplemental file 3.
Supplemental material
Simplified timeline and summary of outcomes for (a) solid tumour cancer participants and (b) haematological cancer participants. HSCT, haematopoietic stem cell transplantation.
Detailed timeline and summary of outcomes for solid tumour cancer participants. *Precise time point is subject to each participants’ course of cancer treatment. NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; MBA20, multiple biochemistry analysis; CBP, complete blood picture; RASS, Richmond Agitation-Sedation Scale; MDQ, Mucositis Daily Questionnaire; ESAS-r-CS, Edmond Symptom Assessment System revised with Constipation and Sleep Disturbance Score; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire; HADS, Hospital Anxiety and Depression Scale; FAACT, Average Functional Assessment of Anorexia/Cachexia Therapy Scale; FACIT-COST, Functional Assessment of Chronic Illness Therapy COmprehensive Score for financial Toxicity.
Detailed timeline and summary of outcomes for haematological cancer participants. Abbreviations. NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; MBA20, multiple biochemistry analysis; CBP, complete blood picture; RASS, Richmond Agitation-Sedation Scale; MDQ, Mucositis Daily Questionnaire; ESAS-r-CS, Edmond Symptom Assessment System revised with Constipation and Sleep Disturbance Score; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire; HADS, Hospital Anxiety and Depression Scale; FAACT, Average Functional Assessment of Anorexia/Cachexia Therapy Scale; FACIT-COST, Functional Assessment of Chronic Illness Therapy COmprehensive Score for financial Toxicity.
Trial oversight, monitoring, auditing and data management
The CANCAN trial is a collaboration by the University of Adelaide, the South Australian Health and Medical Research Institute (SAHMRI) and Central Adelaide Local Health Network (study sponsor). The Data and Safety Monitoring Board (DSMB) will be responsible for the safety of the clinical trial and data monitoring. The DSMB will meet on a monthly basis. Members and responsibilities of the board are summarised in online supplemental table 2.
A data management plan has been prepared using the University of Adelaide’s Research Data Planner Management System. All clinical trial data will be entered directly into REDCap in a de-identified manner and stored on a shared departmental drive with password protection. Any hard copy data will be stored in locked cabinets in secure offices within the recruiting site clinical trial units and SAHMRI, Precision Cancer Medicine Theme, in accordance with international security standards and Australian laboratory accreditation requirements. All documents related to the trial will be stored for 15 years. All study materials and documentation will be readily available for auditing purposes, independent of the sponsor and investigators. All named investigators will have access to the trial data, and there are no contractual agreements that limit this access. Data may be shared upon request and, if shared, will be provided in a de-identified form. Please see online supplemental file 4 for details regarding the planned storage of biological specimens and analyses.
Supplemental material
Patient and public involvement
This project is funded in the Medical Research Future Fund Emerging Priorities and Consumer-Driven Research Initiatives and was assessed by consumers to ensure it was designed in a meaningful manner. The protocol was designed in collaboration with consumer advocacy group, Cancer Voices, with Julie Marker as the consumer representative on our team. Specific aspects of the protocol where consumer engagement was critical in shaping design decisions include the use of PROMs (eg, frequency), specific combination of PROMs, primary outcome measure and strategies to minimise burden on participants who cannot drive during the trial (eg, home-visit nurses). Cancer Voices will also facilitate dissemination of trial results through their national newsletter, social media and other engagement strategies.
Statistical considerations
Sample size
Data for sample size calculations were taken from Keefe et al,37 reporting the incidence of GI-M using the MDQ in people undergoing FOLFOX/FOLFIRI multi-cycle chemotherapy. Calculations were based on the incidence of GI-M and worst diarrhoea. Assuming an overall incidence of GI-M in the placebo group of 70% (combining reported incidences in Keefe et al for FOLFOX + FOLFIRI), a sample size of 176 participants gives 80% power, with two-sided alpha 0.05, to detect an absolute reduction of 21.7% in the incidence of GI-M (ie, incidence in the CBD/THC group of 48.3%). Assuming a mean of 2 (worst diarrhoea) in the placebo group, and SD of 1.5, a sample size of 176 participants gives 80% power, with two-sided alpha 0.05, to detect a reduction of 0.64 points (ie, mean in the CBD/THC group of 1.36).
With this sample size, we are also sufficiently powered to detect changes in QoL (assessed using the EORTC-CLCQ30). Assumed means and SD for the placebo group have been taken from the values reported for the FOLFOX group: the average of the 3-month and 6-month values was taken, along with the higher of the standard deviations (as this will provide conservative estimates).
Statistical analysis
No interim analyses are planned. Statistical analyses will be performed according to Intention-to-Treat principles, with all randomised participants analysed in the treatment group to which they were allocated. All available data will be analysed, including incomplete data from participants who withdraw or do not complete all outcome assessments, unless consent for data use has also been withdrawn. Please see online supplemental file 4 for the complete Statistical Analysis Plan.
Harms
AEs will be identified during each visit using criteria established by the NCI CTCAE, V.5.0. Where parameters are not addressed within the criteria, severity of AEs should be graded as:
Mild: aware of sign or symptom, but easily tolerated
Moderate: Discomfort enough to cause interference with usual activities
Severe: Incapacitating with inability to work or perform usual activities
Life-threatening: Participant is at immediate risk of death
Fatal: Death
The AEs’ relationship with the IP and actions taken will be defined on scales from 1 to 5 (eg, relationship is 1 (definitely attributed to IP) and action taken is 2 (IP adjusted, interrupted, or discontinued)).
The investigator and site staff are responsible for detection, recording and reporting of events that meet the criteria and definition of an AE or serious AE from time of trial registration until 30 days after the administration of the last dose of study medication. All adverse events that occur after the consent form is signed but before clinical trial treatment is administered will be reported by the investigator, if they cause the participant to be excluded from the trial or are the result of a protocol-specific intervention.
Ethics and dissemination
The study protocol was approved by the Central Adelaide Local Health Network Human Research Ethics Committee (2022HRE00037). This protocol has been designed to comply with the Declaration of Helsinki and any subsequent amendments, the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice (CPMP/ICH/153/95) annotated with Therapeutic Goods Administration comments (July 2000), the National Health and Medical Research Council National Statement on Ethical Conduct in Research involving Humans (2007, updated 2018), and the policies and procedures of any applicable local guidelines. This trial has been registered with the Australian and New Zealand Clinical Trials Registry, see online supplemental table 3 for WHO Trial Registration Data Set. At each trial site, the trial will be conducted in adherence with the Protocol, International Conference on Harmonisation, Good Clinical Practice (ICH GCP R2) Guidelines in Australia, and applicable regulatory requirements. When potential participants discuss the trial with their clinician, they will be informed of the study objectives and that participation is voluntary. Each participant will be required to provide consent in either written or electronic form (online supplemental files 1 and 2).
Results of the trial will be published in appropriate peer-reviewed journals and presented at national and international scientific meetings (see Publication Policy; online supplemental file 5). Clinically significant results from the trial will be disseminated among key stakeholders including (1) medical professionals involved in care of cancer patients, via leadership at peak representative bodies (AGITG/ALLG) and other organisations involved will include the Clinical Oncology Society of Australia, the Haematology Society of Australia and New Zealand and the South Australian Comprehensive Cancer Consortium; (2) consumer advocacy groups;38 and (3) supportive care organisations. Our team has a breadth of leadership positions across the public and private sectors with evidence of successful translation to clinical practice and policy, and the capacity to organise follow-up clinical trials to investigate hypotheses generated by CANCAN.
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
The CANCAN team would like to acknowledge Prof Guy Ludbrook for his contribution to the protocol.
References
Footnotes
X @BellasOlivia, @hannahrwardill
OMB and KC contributed equally.
Contributors Conceptualisation: HRW, JBou, SSm, SSh, AZ, TP, GBC, DTY, JBow, JL, JM, GK. Methodology: HRW, JBou, SSm, SSh, AZ, TP, GBC, DTY, JBow, JL, JM, GK. Validation: N/A. Funding acquisition: HRW, JBou, SSm, SSh, AZ, TP, GBC, DTY, JBow, JL, JM. Project administration: HRW, BC, OMB, KC. Investigation: HRW, JB, SSm, SSh, ACWZ, TP, GC, DTY, JBow, GK, OMB, KC. Statistical expertise and analysis: JL. Writing— draft: OMB, KC, BC, HRW. Writing—review and editing: All authors. Ethics and regulatory compliance applications: HRW, BC. Guarantor: HRW.
Funding This work is funded by the Australian Government Medical Research Future Fund (MRFF), awarded $1.49M (AUD) in the Emerging Priorities and Consumer-Driven Research Initiatives (Application Number: 2001877). The IP and placebo will be provided by iX Biopharma who have obtained all relevant licences for importation, distribution, and provision of the study medication. iX Biopharma have also performed all quality assurance tests of the product to confirm its stability at room temperature. This funding source had no role in the study design and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests JBou is CEO and Chief Scientist at See Breeze Pty Ltd, as well as Chief Scientist at Biome Australia Ltd. DTY reports honoraria from Pfizer, Novartis, Bristol Myers Squibb, Ascentage and Amgen; and receives research funding from Novartis. The remaining authors declare no competing interests.
Patient and public involvement Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.