Article Text
Abstract
Objectives Cough occurring in patients with renal cell carcinoma (RCC) was first described in 1935 and is a frequently discussed symptom on patient forums. We aimed to systematically review the available evidence to explore the prevalence and risk factors for persistent cough in patients diagnosed with RCC to establish whether cough could be a presenting symptom of RCC.
Design This epidemiological systematic review used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement 2020.
Data sources Medline, Embase, Science Citation Index, The Cochrane Library, ClinicalTrials.gov and the WHO trials register were searched without language restrictions until 1 June 2023.
Eligibility criteria for selecting studies We included articles of all study designs reporting cough in patients (18 years or older) with RCC attributed to the disease itself or to treatment.
Data extraction and synthesis Data from included articles was extracted using a preprepared and piloted form, and quality assessment was conducted independently by two authors. The risk of bias was assessed in studies other than case reports or case series using the critical appraisal instrument for studies reporting prevalence data. Narrative techniques were used for data analysis and, where appropriate, meta-analysis using a fixed-effects model was performed.
Results Of 509 studies screened, 105 full-text articles were assessed, with 46 papers subsequently excluded, resulting in 59 analysed in depth. There were 105 patients with RCC reported as having a cough due to the disease itself within 30 case reports and 8 case series. When present, most coughs were described as persistent and dry in nature. The cause of cough was attributed to various aetiologies including pulmonary and endobronchial metastasis and paraneoplastic syndromes. Studies reporting patients with RCC developing a cough because of systemic treatment were heterogeneous. Two studies with 238 patients on temsirolimus and 230 on interferon-α (IFN-α) were suitable for meta-analysis using a fixed-effects model. Patients on temsirolimus were more likely to develop a cough than those on IFN-α (OR 1.95 with a 95% CI of 1.05 to 3.63, overall effect Z=2.12 (p=0.03), I2=0%).
Conclusion Cough can occur in patients with RCC, as part of the disease pathogenesis, as an adverse effect of systemic treatment or due to unrelated causes such as pre-existing conditions (eg, asthma). Further research is required to determine the true prevalence and cause and to assess whether cough could be a presenting symptom for RCC.
PROSPERO registration number CRD42022302962.
- Systematic Review
- Thoracic medicine
- Pulmonary Disease
- ONCOLOGY
- Kidney tumours
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A comprehensive literature search (without language restrictions) was performed to identify all the relevant studies.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement 2020 was followed.
The narrative synthesis approach enabled the description and tabulation of patient and disease characteristics.
Narrative synthesis was also used to identify the proposed aetiologies of the cough in patients having a cough due to renal cell carcinoma.
Meta-analysis was limited due to the heterogeneity of the studies.
Introduction
In 2020, there were 431 288 cases of kidney cancer diagnosed globally and an estimated 179 368 deaths from kidney cancer.1 Renal cell carcinoma (RCC) is now the seventh most common cancer in the UK with an estimated lifetime risk of 3% for men and 2% for women.2 Of concern, the incidence of RCC has increased 88% (94% in women and 77% in men) since the 1990s and currently there are approximately 13 300 new cases and 4600 RCC deaths annually in the UK alone. The rate of RCC is expected to rise by a further 26% from 2014 to 2035.3 Many RCCs are diagnosed as an ‘incidental finding,’ with almost 60% of RCCs diagnosed on abdominal imaging used to investigate other conditions.4 Only one-third are diagnosed on the basis of the ‘classic’ symptoms including haematuria, flank pain, a mass in the abdomen and a varicocele. At all stages, RCC may produce hormone-like or cytokine-like substances resulting in paraneoplastic syndromes which could result in hypertension, anaemia, cachexia, weight loss, fever, hypercalcaemia and polycythaemia.5 Some of these substances, for example, prostaglandin E2 (PGE2), are known to be involved in the cough reflex.6 One-third of all RCCs are stage 4 at presentation, spreading to the lungs, brain, bone and liver and so can present with persistent cough, haemoptysis, abnormal liver function tests and bone pain.7
Chronic cough (a cough lasting 8 weeks or more) affects approximately 10% of the adult population and is a common reason why patients visit their general practitioner.8 A cause for the cough is identified in approximately half of cases (eg, asthma, bronchiectasis, gastro-oesophageal reflux or a side effect of medication).9 This means almost half of patients have a chronic cough without an attributed cause.
The recognition of cough occurring in patients with RCC was first described in 1935 by Creevy,10 but only a few clinicians seeing patients with RCC or cough are aware of this. The aim of this systematic review is to summarise the available evidence about the prevalence of cough in patients (18 years or over) with RCC, either attributed to the disease itself or due to the systemic treatment used in these patients. This may establish whether cough could be a presenting symptom of RCC, which may lead to an earlier diagnosis of RCC in some patients.
Methods
A systematic review of the literature from primary studies on cough in adult patients (>18 years old) diagnosed with RCC was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 2020.11 The prospective systematic review protocol was registered on PROSPERO (CRD42022302962). The variations from the protocol included the use of Covidence rather than EndNote software to record the electronic searches, remove duplicates and scan titles to remove any irrelevant work; the quality assessment of all included studies was conducted using the relevant Joanna Briggs Institute (JBI) critical appraisal tools and not for cross-sectional studies as in the protocol, and risk of bias was assessed using the critical appraisal instrument for studies reporting prevalence data instead of Quarterly Income Preferred Securities (QUIP).
Search strategies and selection criteria
A comprehensive systematic literature search was conducted to identify all published studies on the prevalence and persistence of cough in patients (>18 years old) with RCC. Any non-English language papers were translated (using Google Translate) and assessed fully for potential inclusion in the review, as appropriate. The authors did not apply any design restrictions, and all study designs were included (eg, observational/case/cohort studies, non-randomised and randomised controlled trials).
The following electronic databases were searched: Medline, Embase, Science Citation Index, The Cochrane Library, ClinicalTrials.gov and the WHO trials register from their inception to the search date (completed on 1 June 2023), to identify potential studies using a combination of controlled vocabulary and free-text terms (see online supplemental table S1).
Supplemental material
Furthermore, the reference lists of the included studies to identify other relevant studies, the ‘related search’ function in Medline (PubMed) and Embase (OvidSP) and ‘citing reference’ search (to search for articles that cited the included studies) in Science Citation Index Expanded and Embase (OvidSP) were reviewed.
The titles and abstracts of the publications were screened independently by two review authors (WS and JS) followed by full-text screening and data extraction in the same manner. Any differences were discussed and mutual agreement obtained. The reasons for exclusions were recorded and reported in a PRISMA flow diagram. Where appropriate, the authors of published trials were contacted for further information and unpublished data. The Covidence software was used to record the electronic searches and to screen for duplicates, and the two studies found to be suitable for meta-analysis were uploaded to RevMan.
Data extraction and analysis
Data were extracted using a preprepared and piloted form. If overlap of participants between multiple reports was suspected due to common study authors or centres, the authors were contacted for clarification. Variables extracted included the: Study characteristics (authors, year of publication, institution, single vs multicentre, country, language of publication, study period, study design, number of patients enrolled); patient characteristics (age, gender, ethnicity, other comorbidities); tumour characteristics (size of primary tumour, tumour subtype, stage of disease); cough characteristics (nature, duration prior to diagnosis, if cough was thought to develop due to the RCC itself or treatment of RCC and if the cough resolved or persisted following treatment); interventions (no treatment, surgery, embolisation, medical treatment including immunotherapy).
The quality assessment of all included studies was conducted using the relevant JBI critical appraisal tools.12–14 These have a variable number of questions depending on the type of publication, and these were ranked as yes (Y), no (N), unclear (U) or not applicable (NA) as recorded in online supplemental table S2. Further information was sought from study authors where necessary. No study was excluded based on the quality assessment scores since it provided information on the current level of evidence. The risk of bias was assessed in studies other than case reports or case series using the critical appraisal instrument for studies reporting prevalence data.13 The online supplemental table S3 shows that the studies had overall scores of 7 or more (out of 9).
Narrative techniques were used for data analysis, with summary median and quartiles calculated when appropriate. For the remaining analysis, only studies that had sufficient information to calculate the effect measures were included. Subgroup analysis was performed for the histological subtype, stage of disease (size of the primary tumour and whether metastases were present or not) and the treatment received. A meta-analysis using ORs was performed when the types of participants, risk factor definitions and the outcome definitions were similar across at least two studies. 95% CIs were used for deciding whether the risk factor had prognostic value, and I² and χ² tests were used for assessing heterogeneity. The I² value was interpreted as per the Cochrane Handbook guidance, and in the absence of heterogeneity or if the I2 value was under 40% the fixed-effects model was preferred.
Patient and public involvement
Patient and public involvement (PPI) representatives from Kidney Cancer UK formed the PPI support group for the study ‘Cough in patients with suspected or confirmed kidney cancer’ and met every 6 months to review progress and discuss findings. Each meeting was followed by a newsletter, coproduced with the PPI group and disseminated to Kidney Cancer UK.
Results
Study selection
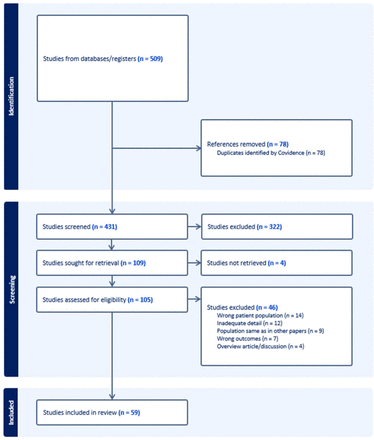
The selection process is presented by the PRISMA diagram (figure 1). Of 509 reports initially identified, 78 were duplicates and were removed. Following title and abstract screening, 322 were identified as irrelevant (eg, on other diseases, such as sarcoidosis or follicular lymphoma, or on quality of life or non-cough aspects of RCC). Of the remaining 109 studies, four papers could not be sourced despite extensive efforts. A total of 105 publications had a comprehensive full-text evaluation, and 46 papers were subsequently excluded at this stage (14 were due to the wrong population, eg, patients with inherited syndromes at risk of developing RCC; 12 due to insufficient detail, eg, description of cough present in 7 of 11 patients with various cancers but no detail if 2 patients with RCC had a cough). Nine papers were from papers describing the same study population (eg, RECORD1 study), seven did not discuss cough as an outcome (eg, article discussing paraneoplastic syndrome but cough not mentioned) and four were overview articles/discussions, focused on the cause of pulmonary manifestations in patients with RCC.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 flow diagram for study selection.
A total of 59 studies met the inclusion criteria.
Cough attributed to RCC itself
There were 38 publications related to cough due to the RCC itself, and these were conducted in the following countries: 9 in the USA, 6 in Japan, 4 in Canada, 4 in India, 3 in the UK, 2 each from China, Germany and Turkey and 1 each from Australia, Egypt, Malaysia, Morocco, Portugal and Singapore (online supplemental table S4 shows an overview of included studies).
Of these, 30 articles were case reports and 8 were case series. There were no systematic reviews or higher level of evidence studies on cough due to RCC itself. Creevy10 referred to cough occurring in 9 of 92 patients in his case series while Zhang et al 15 described 11 of 1326 having a cough at presentation attributed to presumed lung metastases. Doğan et al 16 reported 5 of 11 patients with RCC and endobronchial metastasis having a cough. These were retrospective case series and did not indicate whether the review was of consecutive patients and so the prevalence cannot be deduced. Online supplemental table S4 shows the characteristics of the studies included in this section.
Online supplemental table S5 shows that, in some papers, the age and sex of the patients were not provided. Of the publications describing the age, the range was from late 30s to late 80s, and there was a predominance of male patients (where sex is reported) as would be expected, since RCC has a male predominance.
The nature and duration of cough prior to diagnosis of RCC
Table 1 shows information from 16 papers describing the nature and duration of cough prior to presentation and diagnosis of RCC in 28 patients, where 16 were men, 11 were women and 1 where gender was not stated. The age range was from mid-20s to mid-80s. Nearly all describe the cough as being persistent and dry. Sullivan described a female in her early 60s who presented with an 8-month history of a cough with ‘very little phlegm and just aggravating’. Eventually, it became intractable, occurring ‘any time during the day or night, except when I was sleeping’. The cough described has features of cough hypersensitivity since it was worse with exertion, cold air and talking.17 Tatzel and Sener describe a woman in her late 60s with a 3-month history of ‘persistent, gradually worsening cough, which on occasion woke her from sleep’.18 The duration of the cough prior to the RCC being diagnosed ranged from ‘recent onset persistent cough’,19 from 2 weeks20 to over 2 years.21 Most had a cough that lasted for 3 months.18 22–25
The nature and duration of cough prior to diagnosis of RCC with the corresponding patient and disease characteristics
Cough attributed to the treatment of RCC
The 22 publications reporting cough related to treatment of RCC were from the following countries: 6 from the USA, 4 from multiple nations, 3 from Japan, 2 from Spain and 1 each from Canada, Croatia, Germany, Italy, Israel, Serbia and the UK as shown in online supplemental table S6.
There were five case reports, seven case series, four non-randomised experimental studies and six randomised controlled trials (online supplemental table 6). Table 2 indicates that the study size varied from 1 to 903. One publication with 86 participants did not indicate the age of the patients,26 and another study did not indicate the male to female proportion in the 60 participants.27 Excluding these studies in the subgroup analysis for age and sex, respectively, it was apparent that the age range was from late teens to late 80s with 73% male participants (2236 male, 811 female). The significant male preponderance reflects that the incidence of RCC is higher in men than women.
Patient characteristics and intervention in studies included in the review on cough attributed to treatment
These studies were not designed to determine the prevalence of cough, but cough was mentioned to be present or absent in the participants. Overall, the incidence of cough in patients receiving systemic treatments for RCC (excluding Bukowski et al, who only provided the SE28 and Esteban-González et al who described the presence of cough in terms of treatment cycles rather than patients29) was 13.48% (262 of 1943 participants). Systemic treatments included the use of 2'-deo-oxy-5-fluorouridine-infusion (one report), inhaled interleukin 2 (three reports), sorafenib (two reports), sunitinib (two reports), temsirolimus (three reports), interferon-α (IFN-α) (two reports), everolimus (six reports) and nivolumab (four reports) and in one paper each on the use of apitolisib, axitinib, ipilimumab, tivozanib, pazopanib and, most recently, pembrolizumab. This reflects the recent development of new systemic and immunotherapy agents.
Subgroup analysis
Data on the subtype of RCC
Information regarding the diagnosis of clear cell, papillary or chromophobe RCC was limited since most of the studies did not specify the subtype of RCC. Of the 105 patients who developed a cough due to the disease itself, only 17 patients were confirmed as having clear cell type of RCC,18 23 25 30–36 3 with papillary RCC37–39 and 2 with non-clear cell renal cell carcinoma (ccRCC).40 41
Data on the size of the primary tumour
Data on the size of the primary tumour are documented in online supplemental table S5. The size was not available for 71 of 105 patients with cough at presentation (2 of these patients had excision of the primary tumour 5 years and 32 years previously).42 43 Data were present for 3 patients with T1a,25 34 10 with T1b,17 19 23 25 32 41 6 with T2a,18 24 25 40 44 3 with T2b,24 35 45 8 with T324 31 36 38 46 47 and 4 with T4 tumours.24 33 37 48 This suggests that cough can occur in some patients with small T1 tumours, when the cancer is still at an early stage.
Data on the presence or absence of metastases in patients with RCC who had a cough at presentation
Of 105 patients who had a cough at presentation, 16 patients had no metastases identified, 86 patients were identified as having metastases, 2 had a synchronous lung primary23 34 and in 1 study, the presence or absence of metastases was not discussed.20
Metastatic disease was reported in 86 patients, and it is apparent that RCC can metastasise to a number of different sites within the body. 26 patients had pulmonary metastases,10 15 32 35 36 41 46 48 1 of whom also had liver metastasis.40 12 had endobronchial metastasis,16 42 49–51 3 patients had cardiac metastases,20 37 52 2 had spinal involvement17 24 and 1 patient each had lymphangitis carcinomatosis,47 metastasis in the larynx,53 femur24 and pleura43 and pleura with brain metastasis.54 The site of metastatic disease was not reported in 37 patients.
Impact of metastases on resolution of cough
Of the 28 patients identified as having resolution of their cough, 12 had metastasis present, 15 did not, and in 1 patient it was not stated whether metastasis was present or not.
86 patients were identified as having metastases; however, there was insufficient detail on the 31 patients in the study by Tiwari et al, so these patients were excluded in the analysis.55 Of the remaining 55 patients identified as having metastases, 12 had resolution of their cough. (In 31 patients, the outcome was not stated, 9 patients died and the cough persisted in 3 patients). Of the 12 patients with metastases whose cough resolved, treatment included cytoreductive nephrectomy in 524 and cytoreductive nephrectomy combined with other treatments in 6 patients.17 22 24 46 49 Despite the presence of known metastases, the cough resolved/reduced soon after the nephrectomy in all 11 patients suggesting the primary tumour is responsible in some way for the cough. The twelfth participant’s cough resolved after treatment with only sunitinib.43
The development of cough from the treatment
Some systemic treatments for RCC, including mammalian target of rapamycin (mTOR) inhibitors, tyrosine kinase inhibitors (TKIs) and antiprogrammed death-1 checkpoint inhibitors (ICIs) can result in the development of non-infectious pneumonitis (NIP) in anything up to 45% of patients depending on the class, dose and duration of medication.56–59 Patients with grade 1 NIP are asymptomatic, but cough/dyspnoea is present with grades 2–4 NIP. Only patients identified as having a cough were included in this review. Cough has been reported in 60.7 and 72.2% in patients on everolimus and temsirolimus, respectively;59 however, Levakov et al stated none of their patients with a cough had NIP.27
Only four studies were suitable for OR and p values to be calculated. Motzer et al did not find cough to be found significantly more or less frequently in patients taking everolimus compared with placebo (p =0.398);60 however, Powles et al found cough in significantly more patients on everolimus compared with those on apitolisib (p=0.03).61 Bukowski et al identified that patients treated with sorafenib rather than placebo were less likely to cough (p <0.0001).28
Escudier found that in patients who continued with nivolumab despite disease progression, there was no significant change (p =0.469) in the presence of cough.62
Meta-analysis of studies on patients on temsirolimus and IFN-α developing a cough
Formal meta-analysis was performed on only two studies where the same intervention was measured. It reported the OR of developing a cough in 238 patients on temsirolimus compared with 230 patients on IFN-α, and the outcome is shown in figure 2. The forest plot favours treatment with IFN-α, that is, patients on IFN-α are less likely to have a cough (an adverse event) than those on temsirolimus. There is no heterogeneity (I2 =0%) and so a fixed-effects model was used. The p value of 0.03 indicates that the difference between the two groups is statistically significant.
Cough in patients on temsirolimus versus IFN-α. IFN-α interferon-α; IV, intravenous.
Discussion
This systematic review has explored the current evidence summarising the prevalence of cough in patients with RCC due to the disease itself. No studies or publications were found to directly answer this question. Creevy10 referred to cough occurring in 9 of 92 patients in his case series, Zhang et al 15 described 11 of 1326 patients having a cough at presentation and Doğan et al 16 reported 5 of 11 patients with RCC and endobronchial metastasis had a cough.
This systematic review found 105 patients who developed a cough attributed to RCC. The majority had metastases (86/105). The cause of the cough was mainly attributed to the presence of metastases principally in the lung, endobronchial and intracardiac, or as a symptom manifestation of a paraneoplastic syndrome. RCC was found in all 28 patients (described in table 1) as an incidental finding on a chest CT performed to investigate their cough. These studies report the cough having been present from 2 weeks to over a year before a diagnosis of RCC was made. The cough appears to be a persistent dry cough, with some reporting exacerbations with exertion, cold air and speech, features suggestive of cough hypersensitivity. The resolution of cough following treatment was reported in 28 patients. Interestingly, in 11 patients with metastases, the cough resolved following cytoreductive nephrectomy, suggesting a possible tumour burden effect. Though some renal tumours were large (9.1 cm and 10 cm),18 25 other tumours were small (3.8 cm)25 or located in the lower pole of the kidney,30 suggesting another cause of cough other than through direct diaphragmatic irritation. Embolisation has also been reported to reduce cough, and in these patients the bulk of the tumour remains.38 The hypersensitive nature of the cough described is supportive that cough neural pathways are more sensitive to stimuli, and mediators of hypersensitivity could arise from the primary tumour. Possible mediators include prostaglandins, such as PGE2, which have been found in RCCs63 or interleukin 6.64 Further research is required to confirm a paraneoplastic cause.
Unfortunately, there is insufficient data to determine whether cough is associated with particular histological subtypes of RCC. The limited studies only included patients with ccRCC and papillary RCC, and a focused prevalence study is required to investigate this further.
This systematic review has shown that overall, 13.48% (262 of 1943 participants) reported a cough in patients receiving medical treatment of their RCC. These studies were not set up specifically to find the prevalence of cough, but cough was mentioned to be present or not in the participants. Some studies, however, indicate that treatments, for example, temsirolimus and IFN-α27 65 may be responsible for a cough developing in patients with RCC. Cough may develop in patients on mTORs, ICIs and TKIs if NIP (grades 2–4) occurs.27
Overall, there were limited studies suitable for meta-analysis. The forest plot in figure 2 showed that patients on IFN-α were less likely to have a cough than patients treated with temsirolimus. The cough may develop because of the RCC itself, either from the primary tumour or through metastatic spread. Bukowski et al showed that sorafenib-treated patients reported significantly fewer symptoms versus placebo, including cough (p<0.0001).28
The heterogeneity and nature of the studies identified in this review resulted in some necessary deviations from the protocol registered on PROSPERO (CRD42022302962). We used the relevant JBI critical appraisal tools and not cross-sectional studies as in the protocol, and risk of bias was assessed using the critical appraisal instrument for studies reporting prevalence data instead of QUIP. The lack of awareness of a possible association between cough and kidney cancer is likely to result in under-reporting of this relationship in the literature.
Conclusion
This systematic review suggests that there may be an association of cough in patients with RCC, which typically resolves following treatment of the primary tumour. There is insufficient evidence to determine the prevalence of cough in patients due to the RCC itself, and a prospective study to evaluate this is currently ongoing.66 Should a causal link be determined, the utility of cough as a potential symptom of kidney cancer can be further explored, improving diagnosis and enabling earlier treatment for kidney cancer.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We acknowledge the contribution of Professor Kurinchi Gurusamy for input to the design of the systematic review published on PROSPERO registration number CRD42022302962.
References
Footnotes
X @wendysmithent, @drimranomar
Contributors Study concept and design: WS. Acquisition of data: WS and JS. Analysis and interpretation of data: WS, JS, MIO and MGBT. Original draft: WS. Writing— reviewing and editing: WS, JS, HKP, ML, MIO, FM, SM and MGBT. Study supervision: MIO and MGBT. All authors had full access to the data and take responsibility for the integrity of the data and accuracy of the analysis. WS is guarantor. All authors read and approved the final manuscript.
Funding This work was supported with awards from The Kidney Cancer Research Foundation (Award 177365) and ENT UK Research Foundation (ENTUKFRG014).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.