Article Text
Abstract
Objective Malaria is a major public health concern in most developing countries, with children under 5 years being mainly at risk. We investigated the contribution of individual and community-level covariates to the risk of malaria infection (treatment with artemisinin-based combination therapy for fever or tested positive for malaria via a rapid diagnostic test within 2 weeks prior to the survey) in children under 5 years in Ghana.
Design Population-based secondary cross-sectional study on the 2019 Ghana Malaria Indicator Survey
Setting Ghana.
Participants and methods Secondary malaria data on 3004 mothers and their children under 5 years from the recent 2019 Ghana Malaria Indicator Survey were analysed. Bayesian multilevel modelling under Hamiltonian Monte Carlo is applied to malaria data.
Results The results indicate a weighted malaria prevalence of 29.7% (95% CI: 0.28 to 0.31) among children under 5, and nearly 10% (8.9%) of the risk of malaria infection significantly varied by community differences. The average annual rainfall positively correlates with the prevalence of malaria in a community, while temperature and the built-population index inversely influence it. At the cluster level, the average annual rainfall significantly increased the risk of malaria infection among children under 5 years (adjusted OR (aOR)=17.46, 95% CrI: 1.86 to 167.34). Malaria infections among children under 5 are attributed to household/individual and community-level characteristics. Children from rich households (aOR=0.66, 95% CrI: 0.50 to 0.87), who sleep under insecticide-treated nets (ITNs) (aOR=0.79, 95% CrI: 0.65 to 0.95) and are not anaemic have significantly reduced the risk of malaria infection than those from poor households, children with severe anaemia and those who do not sleep under ITNs at night. Children under 5 years from Gurma (aOR=1.82, 95% CrI: 1.92 to 2.86) ethnic backgrounds are linked to a high risk of contracting malaria, while those from the Mole-Dagbani (aOR=0.70, 95% CrI: 0.51 to 0.98) and Grusi (aOR=0.55, 95% CrI: 0.32 to 0.93) ethnic groups have significantly reduced the risk of malaria infection. All other considered factors were not significantly associated with malaria risk among children under 5 years in this study.
Conclusion Malaria remains a serious health burden to children under 5 years. These findings call for individual and community-level measures, including improved sanitation and preventive education campaigns, to help reduce malaria infections among children under 5 in Ghana, to mitigate malaria infections among children under 5 in Ghana, thereby promoting their health and quality of life (Sustainable Development Goal 3).
- EPIDEMIOLOGY
- Malaria
- Observational Study
- PUBLIC HEALTH
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. The data analysed in the study are available in a public open-access repository. The 2019471 GMIS data for this study were obtained with approval from the DHS program. DHS data files are generally available and accessible after a simple registration process. Further details on how to access the DHS data are available at https://dhsprogram.com/data/Access-Instructions.cfm.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The multilevel modelling framework quantifies disparity in the risk of malaria infection among under-5 children due to the clustering effects.
Multilevel models examine the contribution of both community and individual-level factors on malaria risk among children under 5.
Nationally representative cross-sectional data ensure validity of generalisations of study findings to all children under 5 years.
The study is limited in scope due to a lot of missing or incomplete values at cluster-level covariates.
Not all children who were treated with artemisinin-based combination therapy were confirmed by a malaria test in the laboratory, and, therefore, the malaria prevalence reported in this study may differ from what is reported in the Ghana Malaria Indicator Survey (GMIS) 2019 report.
Introduction
Malaria remains a serious public health concern that accounts for most of the morbidity and mortality cases worldwide, and more particularly in sub-Saharan Africa (SSA).1 Malaria is caused mainly by the Plasmodium parasite, mainly spread in human populations through the bite of the female Anopheles mosquito.1 2
Although malaria is a curable and preventable disease, it remains and continues to have devastating effects on human health worldwide, especially among children and women in developing countries.2 3 The burden of malaria infection affects population groups disproportionally.4 In recent reports by the WHO in 2019 and 2022, the burden of malaria is more prevalent among children, pregnant women, travellers and persons with HIV or AIDS. Again, the WHO reported that out of the 247 million malaria cases and 619 000 deaths worldwide in 2021, the African region alone is responsible for 95% and 96% of cases and deaths, respectively.4 Furthermore, children under 5 accounted for 80% of all malaria deaths in Africa.4 The high number of malaria cases recorded in a country has significant negative effects on the country in several ways. These adverse impacts of the malaria burden are multifaceted and impede the country’s efforts to achieve national and sustainable development goals. The economic and human costs of malaria are numerous and primarily result in the loss of productive hours and household income due to the cost of malaria infection treatment or the inability to work.5 6 Furthermore, the direct and indirect costs associated with malaria result in a vicious poverty cycle in poor households. Thus, in addition to the health burden of malaria, it hinders growth and development, particularly in malaria-endemic countries, including Ghana.7
In most of the malaria-endemic regions, mainly in Africa, malaria infections are higher in rural areas than in urban areas, which may be attributed to poor housing quality, inadequate drainage systems and higher vector density associated with rural settings.8 Malaria risk factors in human populations range from socio-economic, demographic, environmental to climatic conditions. Studies have examined the link between the risk of malaria incidence and socio-economic and demographic characteristics such as poor sanitation conditions in households and staying outdoors to play in the night.8 Moreover, Mafwele9 and Mohammadkhani et al 10 observed significant contributions of climatic conditions, especially temperature and rainfall, to the transmission of malaria in human populations. Furthermore, significant changes in climatic conditions, particularly temperature and rainfall due to human activities, have affected the dynamics of malaria transmission dynamics in SSA.9 11
Malaria infections in Ghana are generally perennial and widespread, with significant differences associated with variations in seasons.12 Moreover, malaria remains endemic across all regions in Ghana, with prevalence ranging between 3% and 31%, and it continues to be a significant cause of childhood morbidity and mortality.13 The association between malaria and deaths of children under 5 has negative implications for Ghana’s efforts at achieving the Sustainable Development Goal 3 (SDG 3), specifically target 3.2, which aims to end all avoidable deaths of neonates and children under 5 years by 2030.14 15 The dynamics of malaria transmissions in Ghana also vary due to differences in geographical regions and climatic factors like temperature, rainfall and humidity.16 Existing literature shows that a high number of malaria infections are often recorded after high rainfall as in most African regions.17
Despite the numerous studies on malaria incidence and its associated risks in Ghana, most of these studies18–21 have usually focused on the demographic and socio-economic risk factors for malaria incidence among children below 5 years. The occurrence of malaria infections among children under 5 remains a serious health threat to their growth, development and survival.22 Furthermore, due to the disproportionately high share of malaria burden among children under 5 years in SSA including Ghana, investigating the contribution of attributes at the individual and group (community) level to the risk or likelihood of malaria incidence among children under 5 in Ghana is limited in the literature. This study aimed at modelling the incidence of malaria among children aged under 5 years as a function of covariates at the community and individual level within the Bayesian multilevel framework that will present new insights for malaria eradication fights in Ghana. The multilevel modelling framework was applied in this study due to the hierarchical structure of the malaria indicator survey data to account for the nesting structure inherent in the data to avoid obtaining biased estimates and subsequent erroneous conclusions.23 24 Moreover, multilevel models (MLMs) can showcase contextual differences and effects across different communities that could be undetected using traditional models.25 Additionally, the Bayesian modelling approach is flexible and powerful and allows for the incorporation of prior knowledge into parameter estimation, yielding estimates with greater precision compared with the frequentist approach.26–29 The results of the Bayesian model are compared with the results from the maximum likelihood estimation (MLE) for precision and reliability of parameter estimates. The results of the study will further help Ghana to accelerate efforts to ensure quality health for all, including children who are most vulnerable to infections such as malaria, and, more importantly, the results of the study will provide further useful information for malaria control in Ghana.
Methods
Data and study design
The analysis for this study was performed on data from the 2019 National Malaria Indicator Survey (2019 MIS) collected by the Demographic and Health Survey (DHS) programme. The DHS in Ghana was carried out through collaborative efforts of the Ghana Health Services, the Ghana Statistical Service and the Ministry of Health, with financial and technical support from ICF International through the DHS programme.30 The two-stage sampling procedure was used in the nationwide survey. These involved the initial selection of 200 enumeration areas (EAs) called clusters and the subsequent random selection of households (HHs) and reproductive women (15–49 years) eligible for interview, and children below age 5 (6–59 months) for malaria and anaemia tests with the consent from parents or caregivers.30 The DHS data set contains geocoded information for clusters and not households or individuals. In this study, a sample of 3004 mothers and their children is analysed. Data collection was carried out in all 200 clusters from 25 September to 24 November 2019 after the household listing exercise that was carried out from 24 June to 10 August 2019.30 The study sample of 3004 children is considered a representative of the population of children below 5 years in Ghana. Moreover, sampling weights were employed to ensure representativeness of survey results at the national level due to possible differences in response rates across urban and rural areas. The data extracted for this study were assessed on 14 March 2023 and did not directly involve respondents (women and children below age 5). Data variables extracted include malaria status (fever within the last two weeks or positive test result for malaria via a rapid diagnostic test) as the outcome variable, individual-level covariates (age group of children (months), anaemia status of child, child slept under insecticide-treated net (ITN), wealth status of household) and community-level variables such as malaria prevalence, land surface temperature, rainfall, built-population indices of clusters which were extracted from the Geospatial covariates file associated with the 2019 GMIS. Other possible cluster-level variables with at least 20% missing values were excluded from this study. For individual and household-level variables, only variables with complete data were used, resulting in a complete-case analysis study. Details of the outcome and covariates at the individual/household and community levels are given below. The set of individual and community-level covariates for this study is based on the literature; age of child,19 20 31 place of residence,31–33 wealth status,32–34 anaemia status19 20 and ownership and use of ITNs (households of children under 5 years with at least one ITN as solicited by the DHS and reported in the 2019 GMIS report)35 36 and the completeness of the information in the survey. The data for this study were accessed from the DHS programme.37
Study variables description and classification
Dependent variable
Malaria status of children below 5 years is the dependent variable for this study. Children who tested positive for malaria based on a rapid diagnostic test or were treated with artemisinin-based combination therapy (ACT) within 2 weeks before the day of the interview as yes and those with a negative test and without treatment with ACT as no.
Individual and household-level covariates
Age of child (months) (categorised as ≤12 (ref), 13–24, 25–36, 37–48, 49–60 months), anaemia status (not anaemic, mild, moderate and severe), use of ITN (no, yes), wealth status (poor, middle, rich) and ethnicity (Akan, Ga/Dangme, Ewe, Guan, Mole-Dagbani, Grusi, Gurma, Mande, Others).
Community-level covariates
Malaria prevalence, built-up population index, rainfall and mean temperature.
Operational definition
Anaemia status
Based on the DHS standard, children with haemoglobin  g/L as anaemic, haemoglobin between 100 and 119 indicate mild anaemia, haemoglobin between 70 and 99 g/L represent moderate anaemia and children with haemoglobin below 70 g/L as severe anaemia.
g/L as anaemic, haemoglobin between 100 and 119 indicate mild anaemia, haemoglobin between 70 and 99 g/L represent moderate anaemia and children with haemoglobin below 70 g/L as severe anaemia.
Malaria prevalence
Mean parasite rate of Plasmodium falciparum in children aged 2–10 years within 10 km (rural) or 2 km (urban).
Built-up population index
Measures the extent of development that falls between 0.00 (extremely rural) and 1.00 (extremely urban).
Rainfall
Average annual amount of rainfall (mm) of cluster.
Temperature
Mean surface temperature of the cluster in °C.
Patient and public involvement
No patient was involved in this study.
Statistical analysis
The multilevel modelling
MLMs are used to examine hierarchical data structures, in which observations are nested. The lowest level, level 1, is nested in the higher level of the hierarchy. The different levels in the hierarchical data result in random and fixed effects in a typical MLM. The use of MLMs is recommended for data where there exists a multilevel structure, which is measured by the intraclass correlation coefficient (ICC). The ICC quantifies the proportion of variance in the dependent variable of interest explained by the clustering effects in the communities.38–40 According to Hayes,41 MLMs are recommended when the measured ICC value is at least 0.05. In practice, random effects are specified in a hierarchical model in three forms based on the existing association between variables: (1) random intercept, (2) random slope and (3) random intercept and random slopes.42 In the random intercept model, each grouping variable is allowed to have its own intercept, but an equivalent slope. Thus, the average of each group is different; however, the relationship with the covariates is not different. Random intercept and slope models allow each group to have its unique intercept and association with the covariates.
In this article, the response variable is the status of malaria infection (yes/no), which is binary in nature for each sampled child. We let  represent the probability of the ith child from the jth cluster have had malaria and
represent the probability of the ith child from the jth cluster have had malaria and  refers to the attributes associated with the selected child. It follows that
refers to the attributes associated with the selected child. It follows that 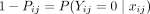 is the likelihood of no malaria infection. The
is the likelihood of no malaria infection. The  , which follows a Bernoulli distribution, is formulated using the logit function as a random intercept and random slope model with variables measured at the individual and cluster levels. The random intercept and slope model accounts for the unobserved heterogeneous effects of both the community and individual/household-level characteristics on the likelihood of under-5 children to have malaria infection or not. The probability that a child will test positive for malaria is not essentially the same for every child sampled from the same group, but the probability is influenced by the characteristics of the child and those of the cluster in which the child lives. The random intercept and slope model for a given child is defined as
, which follows a Bernoulli distribution, is formulated using the logit function as a random intercept and random slope model with variables measured at the individual and cluster levels. The random intercept and slope model accounts for the unobserved heterogeneous effects of both the community and individual/household-level characteristics on the likelihood of under-5 children to have malaria infection or not. The probability that a child will test positive for malaria is not essentially the same for every child sampled from the same group, but the probability is influenced by the characteristics of the child and those of the cluster in which the child lives. The random intercept and slope model for a given child is defined as
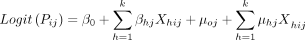 (1)
(1)
where  denote the individual/household and cluster-level covariates for the
k
variables,
denote the individual/household and cluster-level covariates for the
k
variables,  represent parameters for fixed effects and
represent parameters for fixed effects and 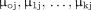 are parameters denoting cluster-level random effects that assume a Gaussian distribution. And
are parameters denoting cluster-level random effects that assume a Gaussian distribution. And  are bivariate normally distributed such that
are bivariate normally distributed such that 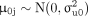 ,
,  and
and 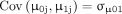 . The likelihood function of (1) is of the form
. The likelihood function of (1) is of the form
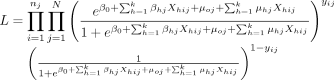 (2)
(2)
Bayesian method of parameter estimation
The Bayesian method of parameter estimation is an alternative method of model parameter estimation which has attracted increased attention in recent times across several disciplines.43 44 The advantages presented by the Bayesian approach are the ability to include background information in the parameter estimation process and its ability to handle complex models often difficult to handle in the classical domain.27 45 The Bayesian method combines the relevant prior knowledge or experience of the investigator, called the ‘prior’ of the observed data, with the likelihood to produce the posterior distribution of the form,  .44 46–48 The Bayesian method is simplified as follows:
.44 46–48 The Bayesian method is simplified as follows:
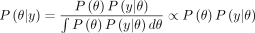 (3)
(3)
where
y
represents the observed sampled data,
θ
a vector of population parameters,  denote the prior distribution and the likelihood of the data,
denote the prior distribution and the likelihood of the data, 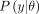 , also known as the generative model or sampling distribution through which the observed data affect the posterior distribution. The posterior distribution,
, also known as the generative model or sampling distribution through which the observed data affect the posterior distribution. The posterior distribution, 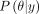 , is often expressed as proportional to the product of the likelihood of the data given the model parameters and the prior distribution.47–49
, is often expressed as proportional to the product of the likelihood of the data given the model parameters and the prior distribution.47–49
Priors within Bayesian methods are specified for each of the parameters and updated through the likelihood of the data to generate posterior distributions. In Liang,48 when data sample sizes are relatively large, prior knowledge is often outweighed by information from the data. Prior distributions can be classified as (1) non-informative priors which are used when there is no sufficient prior information to generate the posterior for valid inferences,50 51 (2) weakly informative priors required in situations where there is little but insufficient information concerning model parameters52 or (3) informative used when expert knowledge regarding the unknown parameters of interest and associated with small variances.44 Given the likelihood of the MLM in (2), the prior is combined with the likelihood of the data to produce the posterior estimates distribution for the model parameters.
The parameters  ,
,  and
and 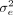 have prior distributions specified as
have prior distributions specified as
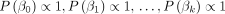 and
and 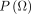 follows the inverse Wishart, IW
follows the inverse Wishart, IW where
Ω
present the variance–covariance matrix,
Σ
is the estimate for
Ω
and
m
is the df of
Ω
. This implies that the prior is diffuse (uninformative) in nature. The Wishart distribution in statistics is a multivariate version of the χ2 distribution for non-integer df and is a generalisation of the Gamma distribution for integer df.53
where
Ω
present the variance–covariance matrix,
Σ
is the estimate for
Ω
and
m
is the df of
Ω
. This implies that the prior is diffuse (uninformative) in nature. The Wishart distribution in statistics is a multivariate version of the χ2 distribution for non-integer df and is a generalisation of the Gamma distribution for integer df.53
The posterior distribution for the parameters  is given as
is given as
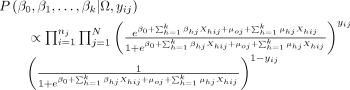 (5)
(5)
Therefore, the complete conditional distribution for Ω is therefore of the form in (6):
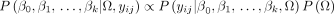 (6)
(6)
The Hamiltonian Monte Carlo approach
The Hamiltonian Monte Carlo (HMC) is an improved Markov Chain Monte Carlo (MCMC) approach that has worked better than the popular Metropolis-Hastings algorithm through the MCMC method, which is based fundamentally on the concepts of Hamiltonian dynamics.54 The HMC basically tries to modify the process in the MH algorithm using two key components: Hamiltonian dynamics and a stochastic momentum (velocity) vector,
ω
. The momentum component is required at any given coordinate position,
θ
. Thus, if  , then a vector of
d
elements is needed for the momentum. The momentum vector examines how to move during the dynamics, and the randomness is a result of the random momentum vector.
, then a vector of
d
elements is needed for the momentum. The momentum vector examines how to move during the dynamics, and the randomness is a result of the random momentum vector.
In the Hamiltonian MC, the two components contained in the Hamiltonian are the potential energy 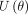 and kinetic energy
and kinetic energy 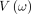 incorporated into the HMC through the Hamiltonian system:
incorporated into the HMC through the Hamiltonian system:

where 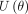 is the potential energy and
is the potential energy and 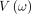 represents the kinetic energy of the system. The leapfrog discretisation integration is applied to obtain good approximations for the Hamiltonian system.54 Further details of how the leapfrog integration technique works are discussed extensively in refs.54–56
represents the kinetic energy of the system. The leapfrog discretisation integration is applied to obtain good approximations for the Hamiltonian system.54 Further details of how the leapfrog integration technique works are discussed extensively in refs.54–56
The HMC technique is, however, dependent on the tuning for the hyperparameters
h
and
L
which are usually user-defined and could lead to significantly high autocorrections between successive iterations if not appropriately defined.39 54 56 To overcome this bottleneck, the No-U-Turn Sampler developed by Hoffman, Gelman and others39 is employed to automatically choose the appropriate values for
L
and
h
in each iteration to maximise the distance at each leapfrog step to avoid random walk behaviour throughout the entire procedure.56 For the proposed model, the parameters
β
, 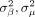 and
and 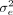 constitute the potential energy,
θ
, and the sampled data represent the kinetic energy. The results of the Bayesian MLMs are validated using expected log predictive density (ELPD), leave-one-out information criterion (LOOIC) and the Pareto k estimate statistic. A higher ELPD value indicates better predictive performance of the model; however, a lower LOOIC value indicates a good model fit and Pareto k estimates<0.5 show fast convergence and stability of posterior estimates.57 In all, four chains with a total of 4000 iterations are used to generate posterior parameter estimates, where the first 2000 iterations are used as burn-in samples. The convergence of the posterior distribution was assessed numerically by the Gelman-Rubin shrink factor,
constitute the potential energy,
θ
, and the sampled data represent the kinetic energy. The results of the Bayesian MLMs are validated using expected log predictive density (ELPD), leave-one-out information criterion (LOOIC) and the Pareto k estimate statistic. A higher ELPD value indicates better predictive performance of the model; however, a lower LOOIC value indicates a good model fit and Pareto k estimates<0.5 show fast convergence and stability of posterior estimates.57 In all, four chains with a total of 4000 iterations are used to generate posterior parameter estimates, where the first 2000 iterations are used as burn-in samples. The convergence of the posterior distribution was assessed numerically by the Gelman-Rubin shrink factor,  ,58 and visually.57 The Pearson’s correlation analysis was used to assess the association between malaria prevalence and each of the cluster-level covariates. The Rao-Scott χ2 test of independence was employed to examine the association between malaria risk and socio-demographic variables. This was performed to adjust for the complex survey design effect and disproportionate sampling of the DHS data set using the survey and Srvyr packages which makes use of sample weights. Multicollinearity among predictors was assessed based on the variance inflation factors (VIFs), with VIF values less than 5 suggesting no significant correlation among model predictors. All analyses were performed in R V.4.1.3.32 R-codes for all analyses can be found at https://github.com/KA-asosega/Bayesian-classical-multilevel.
,58 and visually.57 The Pearson’s correlation analysis was used to assess the association between malaria prevalence and each of the cluster-level covariates. The Rao-Scott χ2 test of independence was employed to examine the association between malaria risk and socio-demographic variables. This was performed to adjust for the complex survey design effect and disproportionate sampling of the DHS data set using the survey and Srvyr packages which makes use of sample weights. Multicollinearity among predictors was assessed based on the variance inflation factors (VIFs), with VIF values less than 5 suggesting no significant correlation among model predictors. All analyses were performed in R V.4.1.3.32 R-codes for all analyses can be found at https://github.com/KA-asosega/Bayesian-classical-multilevel.
Results
From the 3004 under-5 children in this analysis (table 1), the socio-demographic makeup of children under 5 years and their households are presented. This study included nearly an even sex distribution of the children under 5, with 50.4% of males and 49.6% of females. The ownership of ITNs was very encouraging as 86.1% of HHs have at least one ITN compared with 13.9% of those who do not have ITN.
Socio-demographic characteristics and risk of malaria among children under 5 in Ghana
However, only 1936 (64.4%) of children under 5 years of age sleep under ITNs, while 1068 (35.6%) do not sleep under ITNs at night. This suggests that the ownership of ITNs does not necessarily imply the use of ITNs in the study population. Regarding the anaemia status of the children, 1198 (39.9%) were not anaemic, 26.9% had mild anaemia, 31.7% had moderate anaemia and 1.8% were severely anaemic.
The results further show that the majority (60.1%) of children aged below 5 years have some level of anaemia. Over half (53.4%) of the children under 5 years are from poor households, while nearly 20% are from middle-wealth HHs and more than a quarter (26.8%) are from rich HHs. The age distribution of the children shows that 23.7% are 12 months or younger, 19.8% within 13 and 24 months, 20.4% between 25 and 36 months, 19.2% between 37 and 48 months and 16.9% within 49 and 59 months. Of the 3004 children under 5 years of age considered, 2928 (97.5%) indicated their malaria status. Of the 2928 children, 31.7% had malaria and 68.3% had no malaria. It is important to state that the further analysis on the malaria risk will be based on only the 2928 valid cases on the status of malaria in children aged below 5 years. The majority of the children under 5 belong to the Akan ethnic group (34%), followed by Mole-Dagbani (32.4%), Ewe (12.4%), with the Mande ethnic group as the least (1.2%).
The weighted percentage of malaria infection among children under 5 years was 29.7%. Significant variations were observed based on socio-demographic characteristics. The risk of malaria infection was higher among severely anaemic (54%) and moderately anaemic (37%) children compared with those with mild or without anaemia (26%). A high risk of contracting malaria is associated with under-5 children from poor (32%) and middle-wealth (33%) category households than under-fives from rich households (24%). Households with at least one ITN are slightly less likely to contract malaria (29%) compared with children under 5 years in households without ITNs (32%). However, the risk of contracting malaria among under-5 children who sleep under ITNs (29%) is nearly similar to those who do not sleep under ITNs. The analysis further showed that the risk of malaria infection increases with an increase in the age of the child till the third year and declines afterwards as those aged ‘≤12 months’ (25%), ‘13–24 months’ (33%), ‘25–36 months’ (35%), ‘37–48 months’ (29%) and ‘49–59 months’ (25%). A high risk of malaria was observed among children under 5 years of the Gurma (46%), Mande (39%), Ga/Dangme (33%) and Akan (30%) ethnic backgrounds than those of Grusi (20%) and other (21%) ethnic groups in Ghana. Moreover, the χ2 tests established a significant association between malaria infection risk among children under 5 years and anaemia status, household wealth bracket, age of children (months) and ethnic backgrounds.
Cluster-level variables are summarised in table 2. The mean annual malaria prevalence across all 200 EAs was 0.27 and ranged from 0.04 to 0.55. The built-up population had a mean of 0.27 with some very urbanised communities (1.00) as well as very rural communities (0.00). Annual land surface temperature ranged from a minimum of 21.63°C to a maximum of 30.45°C, with an average of 26.14°C across the study area. The log of annual rainfall ranged from 2.80 mm to 30.45 mm and a mean of 2.99 (0.07).
Statistics of cluster-level variables
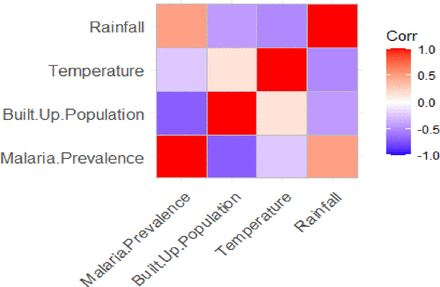
Pearson’s correlation analysis on the covariates at the cluster level showed a significant relationship between malaria prevalence at the community level and the mean annual rainfall, mean temperature and built population index as shown in figure 1. The built population index and the mean surface temperature of the clusters are inversely related to the prevalence of malaria within the clusters. The mean annual rainfall, on the other hand, is positively correlated with malaria prevalence in the community. The results indicate that a high built population index and high mean temperature at the cluster level could reduce the prevalence of malaria, while a high amount of rainfall is associated with a high prevalence of malaria at the cluster level.
Correlation plot for cluster-level covariates and malaria prevalence.
Based on the computed ICC value of 0.088 and a design effect index of 2.32, both satisfy the recommendations of ICC value of at least 0.05 and a design effect index of at least 1.1 in refs. 59 and 60, respectively. Furthermore, the autocorrelations and trace graphs generated as presented in figure 2 established that the posterior distribution of the parameter estimates of the Bayesian MLM converged quickly (mixed very well). The ELPD value for the final model shows an improvement in predictive performance over the null model. Moreover, the low LOOIC of the final model indicates good model fit, and the Pareto k estimates of <0.05 also show that the posterior estimates are stable (converged faster).57
Density plot of the posterior distribution (left panel) and trace plots showing the Hamiltonian Monte Carlo convergence (right panel) of the fitted model.
The Bayesian MLM results for the risk of under-5 malaria incidence are presented in table 3. The results show the significant contribution of individual/household and community-level characteristics to the risk of malaria in under-5 children. At the individual level, the age (months) and whether the child slept under ITN or not significantly influence the chance of malaria infection. The results show that the risk of malaria increases as the child grows in age from 12 months to 5 years.
Bayesian multilevel parameter estimates
The risk of malaria infection for children between 13 and 24 months is 57% (aOR=1.57, 95% CrI: 1.22, 2.01) higher than for children aged 12 months and below. The risk increased further to 88% (aOR=1.88, 95% CrI: 1.46, 2.41) for children aged 25–36 months. The risk of malaria incidence among children aged 37–48 and 49–59 months was 40% (aOR=1.40, 95% CrI: 1.09, 1.80) and 12% (aOR=1.12, 95% CrI: 0.84, 1.48), respectively, higher than children 12 months and younger. Children aged below 5 years who slept under ITNs at night have a reduced risk of malaria incidence by 21% (aOR=0.79, 95% CrI: 0.65, 0.95) compared with children who do not sleep under ITNs.
The results of the Bayesian model further show a significant relationship between the risk of malaria infection and the status of anaemia among children under 5 years. Children under age 5 with moderate, mild and non-anaemic conditions had a reduced risk of 46% (aOR=0.54, 95% CrI: 0.30, 0.96), 64% (aOR=0.36, 95% CrI: 0.20, 0.64) and 67% (aOR=0.33, 95% CrI: 0.18, 0.64), respectively, of being infected with malaria. The risk of malaria incidence among children under 5 from households in the middle-wealth category is not significantly different from those in poor households. However, the risk of malaria among children from households in the rich (upper wealth) category is significantly reduced by 34% (aOR=0.66, 95% CrI: 0.50, 0.87) compared with children below age 5 from poor households.
Moreover, under-5-year-old children from the Gurma ethnic group have an 82% (aOR=1.82, 95% CrI: 1.92–2.86) increased risk of contracting malaria than children of the Akan background. However, children from the Mole-Dagbani Grusi ethnic groups are linked to 30% (aOR=0.70, 95% CrI: 0.51–0.98) and 45% (aOR=0.55, 95% CrI: 0.32–0.93) reduced risk respectively of malaria infection compared with their Akan counterparts.
At the community level, the risk of malaria among children under 5 years is 17 times more likely in children with an increase in mean rainfall (aOR=17.46, 95% CrI: 1.86, 167.34). This could be as a result of the favourable conditions provided for the breeding of mosquitoes that are the main agents of malaria transmission. The relatively low (<5) VIFs for all predictors indicated that predictors are not highly correlated to have any effect on parameter estimates of the model (table 4).
Collinearity test statistics
A comparison of the Bayesian multilevel estimates to those of the classical (MLE) approach (table 5) shows that parameter estimates and odds (risks) are very identical except that parameter estimates and their associated SEs of the MLE approach are relatively smaller (underestimated). Moreover, the credible intervals for the ORs of the Bayesian method are mostly narrower or similar to the CIs of the MLE approach. The relatively narrow intervals from the Bayesian method suggest high precision or reliability compared with estimates from the MLE procedure. However, significantly different estimates and intervals were observed for the rainfall variable.
Maximum likelihood estimation (classical) multilevel parameter estimates
Discussion
In this study, we examined the influence of individual/household and community-level characteristics on the risk of malaria occurrence in under-5-year-old children. The results showed a weighted malaria prevalence of 29.7% (95% CI: 0.28, 0.31). The risk of malaria infection among children under 5 years is significantly lower among children from rich households compared with those from poor and middle-class households. This could be attributed to the fact that children from wealthy backgrounds reside in affluent neighbourhoods, which are often associated with clean surroundings and effective drainage systems, thereby discouraging mosquito breeding.61 In addition, wealthy households have the means to afford and encourage the use of ITNs that significantly minimise mosquito bites, reducing the likelihood of malaria incidence in children below 5 years.62 The findings of this study support earlier results observed elsewhere, where children from rich HHs are associated with a significantly reduced risk of malaria burden.35
The results of the study showed that children under 5 years who sleep under ITNs at night have a low risk of malaria burden in Ghana. This suggests that the use of ITNs is very protective against malaria infection among children under 5 years of age. This may be attributed to the significant reduction in exposure to mosquito bites, as observed in previous studies.18 63 64 However, Isiko et al 65 observed that children under 5 in Nigeria who consistently used ITNs had an increased risk of contracting malaria, which contradicts the findings of this present study. Environmental factors or the over-reliance on ITNs alone as the main preventive measure, while ignoring other malaria preventive strategies could account for the disparity in ITN effectiveness. This further highlights the importance of the effective and proper use of ITNs together with other preventive measures to help in the control and elimination of malaria infections. Enhancing education on the appropriate and effective use of ITNs among parents/caregivers could be intensified to support efforts aimed at combating malaria among children under 5 years, who are particularly most vulnerable to severe illness and death from malaria.
This study’s results indicate that children under 5 who have mild, moderate or non-anaemic conditions are comparatively less vulnerable to malaria than those with severe anaemia. This observation is in line with findings from White’s study,66 which found a significant link between the occurrence of malaria in children under 5 years of age and anaemia status. Moreover, findings of this study collaborate with those observed in previous studies such as Aheto et al 67 in Ghana, Ehouman et al 68 in Côte d’Ivoire and McCuskee and colleagues69 within the African region.
Furthermore, the risk of malaria infection in children under 5 increases significantly from their first birthday to after their fourth birthday (37–48 months). This observation could be linked to the immunity possessed and acquired from their mothers at birth as well as the enhanced care and attention usually given to newborns. This acquired immunity wanes over time, particularly when they are no longer breastfed and are therefore more susceptible to malaria infection.70 Findings in this study corroborate findings observed among children under 5 years in Nigeria.65 This highlights the necessity for age-specific interventions in malaria control and prevention strategies, along with efforts to tailor these measures to specific vulnerable groups and immunity profiles across various age groups.
In addition, the study revealed an association between ethnicity and the risk of contracting malaria among children under 5 years. Children belonging to the Gurma ethnic group faced an elevated risk of malaria compared with Akan children, whereas those from the Mole-Dagbani and Grusi ethnic groups experienced a reduced risk relative to their Akan counterparts. This emphasises the interethnic differences in malaria risk and transmission, suggesting variations in susceptibility to malaria infection and parasite diversity due to genetic differences. The varying risks of contracting malaria associated with race impact initiatives aimed at controlling and eradicating the illness. This underscores the significance of considering genetics in strategies to combat and eliminate malaria, as various ethnic groups may exhibit divergent responses to interventions.71 This finding echoes observations from Burkina Faso, where the Fulani ethnic group has a lower risk of malaria morbidity compared with the Mossi and Rimàibe groups.72–74 Furthermore, understanding the ethnic disparity in the risk of malaria, including immune-epidemiology, could be essential to provide insights into the mechanism of protection or immunity differences to inform control strategies.
Climatic conditions, such as rainfall and temperature, have been established to have significant effects on the incidence of malaria in human populations.9 75 76 Moreover, temperature and rainfall are key drivers of climatic and environmental conditions and are therefore related to malaria spread, especially in the tropics.9 The study results showed that the average amount of rainfall recorded in a community affects malaria transmission by providing suitable breeding sites for mosquitoes, increasing the risk of malaria infections.10 Although the results of the correlation analysis showed a significant association between temperature and malaria prevalence in a community, as observed in earlier studies9 11 by reducing the transmission rate. However, Bayesian multilevel results showed an insignificant reduction in the risk of malaria infection in children aged below five. Moreover, climatic conditions in most SSA countries, including Ghana, have been greatly altered due to human activities, and these have also affected the dynamics of malaria transmission in SSA as a result of the high exposure and vulnerability to climate change coupled with the high malaria burden.11 75
The results of the Bayesian models further provide important information on the fight against malaria in Ghana and other malaria-endemic countries in SSA that share similar climatic and environmental conditions. The significant impact of the wealth of HHs in minimising the risk of malaria incidence among children under 5 years calls for an integrated strategic poverty reduction intervention, especially in rural settings, which could have significant effects on the quality of food and effective use of ITNs. Furthermore, the wealth of households has a significant influence on the decision to sleep under ITN at night as observed in the literature.77–79 Again, the Bayesian MLM produced relatively more precise estimates than the MLE method, which supports findings in ref.80 in which estimates from the Bayesian approach are more precise.
Limitations of the study
Despite the important insights of findings presented by the application of Bayesian multilevel modelling on the malaria incidence dynamics among under-5 children, the study is limited in scope due to a lot of missing or incomplete values at cluster-level covariates. The missing information in some cluster-level variables may limit the findings of study results as some of the excluded variables could have provided further insights on malaria infection among children. Although missing data analysis techniques could have been used to input the missing information, with over 20% of missing values, it could undermine the statistical power of generated results, especially when missing data are not at random. The study used data on children under 5 years, and as such, findings and conclusions are not generalisable to all children, especially those above 5 years. Therefore, the findings of this study should be interpreted with caution as they may not be applicable to other children outside the under-5-year group. Again, not all children who were treated with ACT were confirmed by a malaria test in the laboratory, and therefore, the malaria prevalence reported in this study may differ from what is reported in the GMIS 2019 report. Furthermore, relying on parental- or caregiver-reported fever as a proxy for malaria infection without confirming with a test may be problematic, as fever can also indicate other illnesses in children.
Conclusion
The burden of malaria among under-5-year-old children is very immense and remains a threat to their survival. This study examined the influence of household/individual and community-level characteristics on the risk of malaria incidence among children aged below 5 years through a multilevel Bayesian modelling approach. Bayesian MLM produced relatively more precise estimates than the MLE method. The study showed that children between 24 and 48 months, from rich HHs, belonging to the Mole-Dagbani and Grusi ethnic groups, sleeping under ITN and non-anaemic have a reduced risk of malaria infection. High rainfall at the community level is associated with a high risk of malaria in children aged below 5 years. Continuous sleeping in ITNs, particularly for children below 5 years, should be encouraged among caregivers/parents to minimise malaria infections. Furthermore, personal and community hygienic practices should be encouraged to keep homes and communities free from mosquito breeding hot spots. Effective and strategic poverty reduction interventions in rural settings can significantly help reduce malaria infections in under-5 children. Minimising malaria cases in children under 5 years will promote healthy life and survival of children, which is crucial in efforts to achieve SDG 3 aimed at ensuring quality and healthy populations by the end of 2030.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. The data analysed in the study are available in a public open-access repository. The 2019471 GMIS data for this study were obtained with approval from the DHS program. DHS data files are generally available and accessible after a simple registration process. Further details on how to access the DHS data are available at https://dhsprogram.com/data/Access-Instructions.cfm.
Ethics statements
Patient consent for publication
Ethics approval
The protocol supporting the conduct of the 2019 Ghana MIS was reviewed and approved by the Ethics Committee of the GHS, as well as the ICF International Institutional Review Board. Furthermore, the consent of qualified mothers and their children was obtained properly before conducting interviews and data collection.30
Acknowledgments
We are very grateful to the DHS programme for granting access to the data sets for this study. We are also grateful to the reviewers and the editor for their constructive and insightful comments that have contributed to the improvement of the manuscript.
References
Footnotes
X @Kassim Tawiah
Contributors KAA: conceptualisation, data curation, formal analysis, investigation, methodology, software, validation, visualisation, writing original draft, writing—review editing and guarantor of integrity of the entire study. ENA: conceptualisation, data curation, formal analysis, investigation, methodology, supervision and writing—review editing. AOA: conceptualisation, investigation, methodology, supervision, validation, visualisation and writing—review editing. EO-D: conceptualisation, data curation, formal analysis, investigation, supervision, validation, visualisation and writing—review editing. KT: conceptualisation, data curation, formal analysis, investigation, methodology, validation, visualisation, writing original draft and writing—review editing.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.