Article Text
Abstract
Introduction Postoperative pancreatic fistula (POPF) is the most frequent complication after partial pancreatectomy, which is by definition associated with clinical consequences requiring changes in postoperative management. Despite numerous scientific efforts, effective procedures to prevent POPF are lacking. Obsidian ASG autologous platelet-rich fibrin matrix has been effectively applied to prevent anastomotic leakage following colorectal surgery. This study is the first to investigate the feasibility of using the sealant in pancreatic surgery.
Methods and analysis 25 consecutive patients scheduled for elective formal partial pancreatectomy due to any underlying disease fulfilling the eligibility criteria will be included. Obsidian ASG sealant prepared out of 120 mL of each patient’s whole blood will be applied to the pancreatic stump or the pancreatic anastomosis, respectively. The primary endpoint is the feasibility of the procedure, for example, the proportion of patients undergoing successful trial intervention. Secondary endpoints comprise safety and surgical outcome parameters including rate and severity of POPF as well as further pancreas-specific complications as defined by the International Study Group of Pancreatic Surgery during 90 days after surgery. Patients will be matched with a historic collective in a 1:2 ratio to gain first data on efficacy.
Ethics and dissemination This trial and the associated study protocol (V.1.1.1, date 26 March 2024) were approved by the institution’s ethics committee (reference number 2191/2023). All trial procedures are performed in accordance with the International Council for Harmonisation harmonised tripartite guideline on Good Clinical Practice and the ethical principles of the Declaration of Helsinki. After completion of the study, results will be published in due course.
Trial registration number The trial was registered in the German Clinical Trials Register on 6 May 2024 (DRKS-ID: DRKS00034052).
- Patients
- Pancreatic surgery
- Pancreatic disease
- SURGERY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
One of the strengths of this trial is the careful monitoring of adverse events to gain safety besides feasibility data.
Valid results on the efficacy of the trial intervention will not be obtained due to the feasibility study design with a small number of interventions.
The open-label, non-randomised trial design is prone to several sources of bias, especially selection, performance and detection bias.
Introduction
Rationale of the trial
Formal partial pancreatectomy, that is, distal pancreatectomy (DP) or partial pancreatoduodenectomy (PD), is the treatment of choice for several malignant and premalignant pancreatic diseases including pancreatic cancer and its precursor lesions as well as neuroendocrine tumours.1 2 With the centralisation of pancreatic surgery in specialised institutions and improvements in perioperative management, procedures have become safe with mortality rates below 2% and low failure-to-rescue rates.3 4 However, postoperative morbidity still occurs in 65% of patients and therefore remains unsatisfactorily high.3 The most frequent and relevant complication is postoperative pancreatic fistula (POPF) resulting from healing disorders of the pancreatic anastomosis after PD or leakage from the pancreatic stump after DP.5 POPF affects the patient’s postoperative course by definition as it may cause intra-abdominal infection or erosional bleeding requiring changes in clinical management such as anti-infective treatments, re-interventions and re-operations as well as intensive care unit stay and prolongation of total hospital stay.6 The rates of clinically relevant POPF, that is, POPF grades B/C as defined by the International Study Group of Pancreatic Surgery (ISGPS), reported in literature reach up to 27% after DP7 and 22% after PD8 (even 38% after robotic PD),9 illustrating the unsolved problem of POPF. Prevention of POPF is particularly important in patients undergoing extended pancreatic resection including arterial reconstruction, since these patients are at high risk for life-threatening post-pancreatectomy haemorrhage caused by intra-abdominal enzyme-rich fluid collections.10 11
Preliminary data
Numerous previous studies provided evidence that none of the existing surgical techniques and perioperative measures are effective in the prevention of POPF.12–15 A recent Cochrane review summarises the available evidence on fibrin sealant use to prevent POPF after PD and DP, showing inconclusive results and uncertain evidence on this topic.14 Further research is therefore mandatory, and the evaluation of new approaches is required to solve the hitherto intractable problem of POPF. The thrombocyte-enriched, completely absorbable Obsidian ASG matrix is developed to improve tissue regeneration and healing of gastrointestinal anastomoses by sustained release of an up to eight times multiplied concentration of non-activated platelets and continuous release of growth factors over a period of 5–7 days after surgery.16 Hence, the application of Obsidian ASG may accelerate tissue proliferation, and the anti-inflammatory and antimicrobial platelet properties may offer control of potential contamination, which may be especially important after PD. Obsidian ASG has already been used to prevent postoperative anastomotic leakages in colorectal surgery, and its application has been shown to be safe, feasible and related to low rates of anastomotic leakages.17 18 The application has not yet been demonstrated to be superior to standard therapy. This may be due to the low rates of anastomotic leakage in colorectal surgery and the small number of cases in previous trials. This is the first study to assess the application in pancreatic surgery with the aim to prevent POPF following partial pancreatectomy.
Methods and analysis
This clinical trial protocol is written according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement.19 Adherence to these recommendations is documented in the SPIRIT checklist (see online supplemental additional file 1). The trial was registered with the Clinical Trials Register (DRKS00034052) before the enrolment of the first patient. The trial is scheduled for completion in December 2025, with the results to be published in due course.
Supplemental material
Trial design and trial-supporting facilities
This is an investigator-initiated, single-centre, open-label, phase II clinical trial with one intervention arm and an exploratory study design. There will be a 1:2 matching with patients from a historic collective on the basis of their main characteristics (age, procedures and histopathological findings) in order to receive two comparable study groups. The sponsor of the trial is the Medical University of Vienna, Austria. The sponsor had no role in the design of this study and will not have any role during its execution, analysis of the data, interpretation of the findings or the decision to submit the results for publication. The coordinating investigator is the sponsor’s representative and also the trial’s statistician. The trial will be conducted in close cooperation with the Coordinating Centre for Clinical Trials, Medical University of Vienna, Austria, which is in charge of the trial monitoring, including initiation and close-out visits. The site of this trial is the Department of General Surgery, Division of Visceral Surgery, Medical University of Vienna, Austria.
Trial population
Consecutive patients scheduled for elective, partial pancreatic resection, that is, PD or DP, due to any underlying disease will be screened for eligibility, and informed consent will be reached before the inclusion of the patient. Inclusion criteria comprise ≥18 years of age, ability to understand character and individual consequences of the clinical trial, as well as written informed consent (see online supplemental additional file 3 for the informed consent form). In case of withdrawal of informed consent, patient data will be excluded from analysis. Patients with severe systemic disease that is a constant threat to life, classified as American Society of Anesthesiologists’ score grater than 3, and patients with known hypersensitivity to any component in the formulation of the investigational medical product, as well as those patients with understanding or language problems and patients with an inability to comply with the study and/or follow-up procedures will be excluded. Pregnant and breastfeeding patients as well as patients with concurrent participation in another interventional clinical trial with interference with the trial outcome will also be excluded. For patients with childbearing potential, the presence of a preoperative negative urine or negative blood pregnancy test and adequate contraception until 14 days after trial intervention is therefore required.
Supplemental material
Supplemental material
Trial intervention and perioperative management
Preparation
On the day of surgery, 120 mL of whole blood will be withdrawn from the individual patient. Then, the matrix will be prepared using 300 mg of tranexamic acid and processed through a fully automated Vivostat microprocessor-controlled system (Vivostat A/S, Alleroed, Denmark). The blood will be heated up to 36°C and separated by centrifugation in the upper reservoir chamber of the processing unit. The resulting plasma will be combined with Batroxobin, leading to the polymerisation of acid-soluble fibrin 1. This process will effectively remove excess fibrinogen and thrombocyte-depleted serum, leaving concentrated fibrin 1 and thrombocytes. To dissolve the available fibrin and create a stable clot matrix with high elasticity, tensile strength and crack resistance, the fibrin concentrate will be mixed with sodium acetate buffer (pH 4). The resulting thrombocyte-enriched matrix will then be embedded in a fibrin scaffold.
Surgical procedures
Surgical steps and techniques will be carried out according to the institutional standard procedures such as open, laparoscopic or robotic partial pancreatectomies. After exclusion of distant tumour spread and after confirming local resectability, PD or DP will be performed depending on the localisation of the disease. PD is usually performed openly using an arterial or uncinate first approach and includes the TRIANGLE procedure in patients with cancer. Reconstruction after PD is routinely performed with an omega loop and a double-layer end-to-side pancreatojejunostomy and an end-to-side hepaticojejunostomy, each with 5/0 or 6/0 monofilament atraumatic single sutures. An internal stent is neither recommended nor prohibited. During DP, transection and closure of the pancreas is routinely performed above the portomesenteric axis with any linear stapling device which is selected at the surgeon’s discretion. In case of too thick tissue, transection will be performed with a surgical scalpel followed by separate ligation of the pancreatic duct and suture of the entire pancreatic remnant. If indicated, additional resections may be performed depending on the individual patient’s intraoperative findings. Frozen section specimens will standardly be taken from the pancreatic and bile duct remnant. A ligamentum teres hepatis patch as an additional covering of the pancreatic remnant is permitted but not recommended. Any other additional coverage, except for the investigational drug, is not allowed because there is no evidence, and therefore it would not be the institution’s standard.
Application of the investigational medicinal product
At the end of the surgery, 5–6 mL of Obsidian ASG sealant will be applied to the pancreatic stump in DP or to the pancreatic anastomosis in PD. According to the surgical approach, application systems for open or minimally invasive surgery will be used. After the application of the investigational medicinal product, at least one intra-abdominal drainage tube will be placed and left in place at least until postoperative day (POD) 3 after surgery. Perioperative administration of somatostatin analogues is permitted but not recommended. The administration must be documented in the case report form (CRF; see online supplemental additional file 2).
Supplemental material
Control group
To serve as a basis for further planning of a randomised controlled trial regarding the secondary outcome parameters, the enrolled patients will be matched with a historic collective (age, procedures, histopathological findings) extracted from the pancreatic surgery database of the Department of General Surgery, Medical University of Vienna in a 1:2 ratio.
Risk of bias
The open-label trial design with a single prospective intervention arm and a matched historical control group bears considerable sources of bias, especially selection, performance and detection bias. However, since this is the first trial to use the investigational medicinal product in pancreatic surgery, a small and non-randomised pilot trial focusing on safety and feasibility seems appropriate at this early stage of evaluation of a new surgical intervention.20 Procedures will be standardised and the trial personnel will be informed and trained at the site initiation visit; hence performance bias will be reduced. In addition, adherence to the trial protocol will be controlled by regularly on-site monitoring.
Outcome parameters
Assessment of feasibility
The primary outcome is the feasibility of the trial intervention defined as the proportion of patients undergoing successful trial intervention. The successful application of Obsidian intraoperatively to the pancreatic anastomosis or pancreatic stump will be considered a successful trial intervention.
Assessment of safety
Patients will be closely monitored for the occurrence of adverse events (AE) and serious adverse events (SAE). The incidence of all AE will be ascertained by the investigators using non-leading questions, noted as spontaneously reported by the patients to the medical staff or observed during any measurements on all study days. Only events that occurred after enrolment and during the follow-up will be collected. An SAE is defined as any AE occurring during the observation period that results in death, is life-threatening, requires or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly/birth defect, is otherwise medically relevant and/or requires intervention to prevent any of these outcomes. All SAE must be reported to the Coordinating Centre for Clinical Trials, Medical University of Vienna, Austria, within 24 hours after becoming known.
Secondary outcome parameters
The secondary outcome parameters comprise all relevant surgical complications, that is, clinically relevant POPF,6 delayed gastric emptying,21 postpancreatectomy haemorrhage,22 lymphatic fistula,23 postpancreatectomy acute pancreatitis,24 intra-abdominal fluid collection/abscess, wound infection, burst abdomen, perioperative sepsis,25 reinterventions and reoperations, 90-day mortality, length of intensive care unit and total hospital stay, as well as readmission to hospital. Postoperative complications will be graduated as proposed by the ISGPS and Clavien-Dindo26 as applicable.
Schedule of trial procedures and follow-up visits
As presented in table 1, there will be seven trial visits from screening to the last follow-up at POD 90. Except for the trial intervention on the day of surgery, all procedures including assessment of laboratory parameters belong to the perioperative standard procedures after pancreatic resection. In patients with childbearing potential, a pregnancy test will be performed during the routine preoperative laboratory examinations.
Study visits
Statistical methods
Sample size calculation and timelines
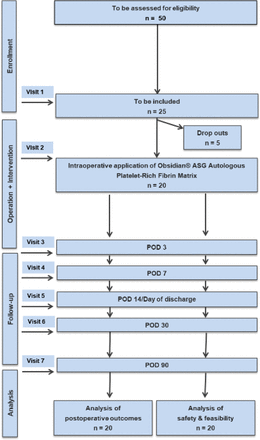
In this a proof-of-concept trial, the focus is on the feasibility and safety of the procedure. Hence, no formal sample size calculation has been performed. We have chosen a number of patients, which is considered valid to obtain first data on the feasibility and safety of the trial intervention. 25 patients are planned to be enrolled in this trial. Considering drop-outs of 5 patients (due to inoperability in case of distant metastasis, local inoperability or in case of total pancreatectomy), the remaining 20 patients will be sufficient to evaluate feasibility. The trial preparation phase started in December 2023. The inclusion of the first patient is planned for May 2024. The duration of the clinical trial for each individual patient will be 3 months. The flow chart in figure 1 illustrates the structure of the trial flow. Nowadays, our centre carries out at least 130 partial pancreatectomies per year. Thus, the time taken to recruit 25 patients out of 50 patients screened for eligibility is expected to be 5 months. Taking into account 2 months for preparation and another 2 months for analysis, the duration of the entire trial will be approximately 12 months.
Trial flow. POD, postoperative day.
Statistical analysis
All analyses will be of descriptive character and will be performed with the software programme SPSS. Quantitative variables will be presented as median and dispersion as IQR or 95% CI. For comparison with the historical control group, the non-parametric Kruskal-Wallis test will be used to compare continuous parameters. For categorical parameters, absolute and relative frequencies will be calculated and compared using the χ2 or Fisher’s exact test as appropriate. Mortality will be calculated using the Kaplan-Meier method. Missing data are expected to be rare, so no imputation will be performed. Two-sided p values will be used and a difference will be considered statistically significant at p<0.05. Interim analyses are not planned.
Data collection and data management
The investigator or a designated representative must enter all protocol-required information in the CRF (see online supplemental additional file 2). The CRF should be completed as soon as possible after the information is collected, preferably on the same day when a trial participant is seen for an examination, treatment or any other trial procedure. The reason for the missing data should be provided. The investigator is responsible for ensuring that all sections of the CRF are completed correctly and that entries can be verified in accordance with the source data. In general, all entries in the CRF must be verifiable by source documents. In advance, exceptions to this rule can be defined by the sponsor. A detailed list will be provided in the Investigator Site File (ISF). Finally, there must be no data that are inconsistent between the CRF and source documents. Completeness, validity and plausibility of data will be checked at the time of data entry. If no further corrections are to be made in the database, it will be closed and used for statistical analysis. Any reason for missing data should be documented. The investigator is responsible for the completeness of data as well as compliance with institutional data management regulations. The investigator will archive all trial data (source data and ISF, including the patient identification list) according to Good Clinical Practice and to local law or regulations. All data shall be made available if requested by relevant authorities.
Monitoring
Monitoring will be done remotely and by a clinical monitor’s personal visits as defined by the Coordinating Centre for Clinical Trials, Medical University of Vienna, Austria. During on-site visits, the monitor will review entries into the CRF on the basis of source documents. Additionally, by remote monitoring and frequent communication, the monitor will ensure that the trial is conducted according to the protocol and regulatory requirements. Therefore, the investigator must allow the monitor to verify all essential documents and must provide support at all times to the monitor.
Ethics and dissemination
This trial was approved by the institution’s Ethics Committee (reference number 2191/2023) on 3 May 2024. Every patient participating in the study will be provided with personal injury insurance, as well as subsidiary medical liability and medical professional legal expenses insurance. All trial procedures are performed in accordance with the International Council for Harmonisation harmonised tripartite guideline on Good Clinical Practice and the ethical principles of the Declaration of Helsinki. Once the study has been completed, the results will be published in due course.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors UK conceived the study and drafted the protocol and the manuscript. CG was involved in the conception of the study and drafted the protocol and the manuscript. CD was involved in the conception of the study and approved the final manuscript. C-SL conducted revisions and approved the final manuscript. SR conducted revisions and approved the final manuscript. KS conducted revisions and approved the final manuscript. MS conducted revisions and approved the final manuscript. OS was involved in the conception of the study, supervised revisions of the protocol and approved the final manuscript. The guarantor is OS.
Funding This study was funded by Medizinische Universität Wien.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.