Article Text
Abstract
Objectives The aim of this study was to compare the cost-effectiveness of total shoulder arthroplasty (TSA) and hemiarthroplasty (HA) and explore variation by age and gender.
Design Cost-effectiveness analysis using a lifetime cohort Markov model.
Setting National population registry data.
Participants Model parameters were informed by propensity score-matched comparisons of TSA and HA in patients with osteoarthritis and an intact rotator cuff using data from the National Joint Registry.
Interventions TSA and HA.
Primary outcome measures Quality-adjusted life years (QALYs) and healthcare costs for age and gender subgroups. A probabilistic sensitivity analysis was performed.
Results In all subgroups, TSA was more cost-effective, with the probability of being cost-effective about 70% for TSA versus 30% for HA at any willingness-to-pay threshold above £1100 per QALY. TSA was dominant in young patients (≤60 years) with a mean cost saving of £463 in men and £658 in women, and a mean QALY gain of 2 in both men and women. In patients aged 61–75 years, there was a mean cost saving following HA of £395 in men and £181 in women, while QALYs remained superior following TSA with a 1.3 gain in men and 1.4 in women. In the older cohort (> 75 years), the cost difference was highest and the QALY difference was lowest; there was a cost-saving following HA of £905 in men and £966 in women. The mean QALY gain remained larger after TSA: 0.7 in men and 0.9 in women.
Conclusion TSA was more cost-effective than HA in patients with osteoarthritis. QALYs were superior following TSA in all patient groups. Cost differences varied by age and TSA was dominant in young patients.
- Patients
- Shoulder
- ORTHOPAEDIC & TRAUMA SURGERY
- Elbow & shoulder
- Health economics
Data availability statement
Data may be obtained from a third party and are not publicly available. Original data can be requested from the National Joint Registry.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Data from the National Joint Registry were used to inform estimates of health utility and cost in matched groups of total shoulder arthroplasties and hemiarthroplasties.
The analysis was separated by age (≤60 years, 61–75 years, >75 years) and gender.
Modelling assumptions were necessary to estimate parameters beyond the 9 years of available follow-up.
There remains a risk of confounding of the relationship between total shoulder arthroplasty and hemiarthroplasty despite matching on propensity scores.
Introduction
Shoulder arthroplasties are increasingly used in the management of glenohumeral osteoarthritis (OA) and the annual costs are substantial.1 2 Shoulder arthroplasties can be classified into two groups; anatomical and reverse prostheses. Total shoulder arthroplasty (TSA) and hemiarthroplasty (HA) are anatomical prostheses, which are used in patients with an intact rotator cuff. Recent population registry studies showed TSA has a lower rate of revision and reoperation and results in superior patient-reported outcome measures compared with HA.3 4 The risk of revision arthroplasty has been shown to differ by patient age and gender, which may result in cost-effectiveness (CE) varying in different groups.5 TSA implants are more expensive and the duration of surgery is longer; however, this initial cost difference may have limited impact over the lifetime of the patient.
The management of glenohumeral OA in young patients is an area of particular uncertainty. This group has the highest rate of revision and reoperation across the patient’s lifetime, and the National Institute of Health and Care Excellence (NICE) recommended an economic analysis of TSA versus HA in patients 60 years and under.6 Economic analyses from North America compared TSA with HA and showed TSA to be more cost-effective to varying degrees.7–9 The parameters were calculated from observational studies and small randomised trials. The data on which to base the utility assumptions were limited, and additional costs of reoperations were not included.
The National Joint Registry (NJR) of England, Wales, Northern Ireland and the Isle of Man includes a large population of anatomical shoulder replacements, data entry commenced in 2012.10 Costs paid for components are collected from hospitals across contributing regions of the United Kingdom to provide a more granular estimate of prosthesis costs. These data provide the opportunity to compare anatomical shoulder arthroplasties within age and gender subgroups. The aim of this study was to determine whether TSA or HA was more cost-effective in the management of glenohumeral OA in patients with an intact rotator cuff and explore variation by age and gender.
Method
The Consolidated Health Economic Evaluation Reporting Standards 2022 reporting guideline was used to inform this report (see online supplemental material).11
Supplemental material
Population characteristics
The study population for estimation of the revision and reoperation parameters included 14 698 anatomical shoulder arthroplasties from a prior study using NJR data linked to Hospital Episode Statistics (HES).4 Data were collected from April 2012 until July 2021. Arthroplasties performed for an indication other than OA or in patients without an intact rotator cuff were excluded. The mean age of the population was 70.1 (SD 9.6), 31.7% men and 68.3% women. The majority had an American Society of Anesthesiologists Physical Status Classification System (ASA) of II or III (ASA I—9.0%, II—67.5%, III—23.0%, IV—0.4%). The population flow diagram is shown in online supplemental figure 1. The number of arthroplasties in each group and the full population characteristics by implant are shown in online supplemental tables 2–5. The level of socioeconomic deprivation was defined in HES12 .
Model structure and perspective
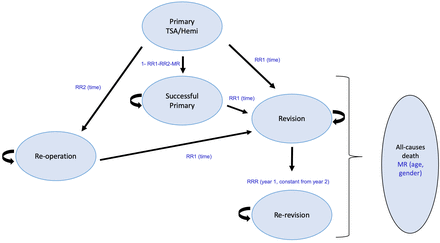
CE analysis was undertaken for hospital costs with a maximum time horizon of 60 years. The time horizon varied according to the gender and age-specific mortality rate of UK life tables. The age for the cohort entering the model varied from 40 to 90 years. A Markov model with time dependency was used, the structure of the model is shown in figure 1. The model simulated a 1000-patient cohort separately for each age and gender. Patients transitioned through a six-state model according to specified transition probabilities representing time-dependent risks for annual cycles (online supplemental tables 6–11). The model structure separated subgroup heterogeneity from parametric uncertainty using subgroups defined by age group and gender as previously described.13 14
Model structure. MR, mortality rate; RR1, revision rate; RR2, reoperation rate; RRR, rerevision rate; TSA, total shoulder arthroplasty.
Patients started with a primary TSA or HA and after a 1-year cycle moved to one of four different health states (2) to (5): state (2) remains in the ‘successful primary’; state (3) ‘revision’ of their primary arthroplasty; state (4) ‘reoperation’; or reached the final state (6). In the next year’s cycle, a new health state (5) ‘rerevision’ was added to capture patients requiring a second revision procedure. The rest of the cohort evolved across states (2) to (4), and (6) according to the transition probabilities. Cycles were repeated until all patients had died within the 60-year time horizon.
Outcomes—revision, reoperation and mortality
The rates of revision and reoperation for HA and TSA were estimated using patient-level data from the NJR. The rates were calculated separately for each of three age groups (1) 60 years or younger, (2) 61–75 years and (3) over 75 years. HA and TSA were matched using propensity scores within each age group to minimise baseline differences in population characteristics using 11 covariates reported previously.4 These included age, sex, American Society of Anesthesiologists Physical Status Classification System (ASA), rotator cuff condition, primary surgeon seniority, assistant seniority, surgical approach, unit type, mean number of anatomical shoulder arthroplasties performed per year by the responsible consultant, Charlson Comorbidity Index and deprivation index. The standard mean difference was <0.1 for each of the 11 covariates. Characteristics of the subgroup populations and details of the matching process are available in online supplemental tables 3–5.
Follow-up data were available for 9 years and modelling was necessary to extrapolate beyond the available follow-up period. Parametric survival models were specified separately to model the implant duration as time-to-event from primary surgery to revision and reoperation for each implant and subgroup, using the Weibull distribution, which allows for increasing or decreasing hazards over time, and it has shown good adjustment to estimate time-to-event clinical outcomes for orthopaedic implants.15 The transition probabilities for each cycle, tp(cycle t), were calculated using the cumulative hazards, H(t), according to the methods described below:
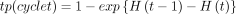
The cumulative hazard for the Weibull distribution is 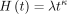 , and the parameters λ (scale) and κ (shape) were estimated for each subgroup. A Weibull regression was used to estimate the hazards. The distribution of time-to-event, T, was a function of gender, age, and the whether the prosthesis was HA. The hazard function of a Weibull regression was modelled as follows:
, and the parameters λ (scale) and κ (shape) were estimated for each subgroup. A Weibull regression was used to estimate the hazards. The distribution of time-to-event, T, was a function of gender, age, and the whether the prosthesis was HA. The hazard function of a Weibull regression was modelled as follows:

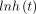 represents the baseline log hazard at time point t, with
represents the baseline log hazard at time point t, with 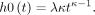 The effect of HA is measured as a multiplicative effect (additive in the log scale) with the estimated coefficient
The effect of HA is measured as a multiplicative effect (additive in the log scale) with the estimated coefficient  , so that the risk of revision or reoperation is larger for HA than for TSA if
, so that the risk of revision or reoperation is larger for HA than for TSA if 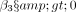 , with the multiplicative effect measured by
, with the multiplicative effect measured by 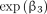 .
.
The rate of rerevision following a successful revision was taken from a meta-analysis.16 The transition to death was considered as all-cause mortality, measured from the most recent 2018–2020 UK life tables, as no deaths were observed during surgery.4 The life tables present the mortality rate for each age separately for men and women. The mortality rate from age 40 to 100 was used as the transition probabilities to death for each age within each one of the three age groups.
Outcomes—health-related quality of life
Oxford shoulder scores (OSSs) from a previous population-level comparative study were used to estimate health-related quality of life (HRQoL).3 17 The results were skewed towards the highest score and the median score was used for the purpose of the quality of life estimations.3 The OSSs were mapped to the EQ-5D-5L.17 There was minimal change in the OSS from 6 months to 5 years.3 The model addressed the postoperative recovery period in year 1 by halving the improvement in the EQ-5D-5L value from baseline for the first 6 months followed by the full EQ-5D-5L for the second 6 months. There was no further change in HRQoL after 1 year.
Reports of shoulder scores in revision arthroplasty are very limited.16 Revision utilities were estimated as 15% less than the combined TSA and HA EQ-5D values. This estimate was made from a combination of data in shoulder and knee arthroplasty.14 16 The same trajectory of improvement in HRQoL in the first year was applied to the revised state. HRQoL following rerevision was assumed to fall by the same proportion as it did from primary to revision arthroplasty. Full details of HRQoL estimations are included in online supplemental table 1.
Cost estimates
The primary source of information for cost estimation was hospital reimbursement values for shoulder procedures from the 2022/2023 National Tariff Payment System using Healthcare Resource Group (HRG) codes.18 The codes do not differentiate between the two types of anatomical shoulder arthroplasty. Two key elements of the total cost of each procedure were used to estimate the difference between primary HA and TSA: length of the procedure and component costs. Costs are described in the British Pound (£). Data from the NJR EMBED price benchmarking service were used to calculate the mean price of TSA and HA components.19 The HRG code HN52 Very Major Shoulder Procedures for Non-Trauma was used as the baseline cost for HA and TSA.18 Theatre time costs were calculated using estimated durations of surgery combined with theatre time cost estimations, the full calculation is available in online supplemental figure 3.20 21 The total difference in cost was halved and added to the HRG value to estimate the TSA cost and subtracted from the HRG value to estimate HA cost. This meant the mean cost of HA and TSA was equal to the NH52 code value. See online supplemental table 14 for further information and the individual values.
The cost of a revision and rerevision arthroplasty was estimated from relevant HRG codes and assumed to be equal between the groups. The model did not include community costs, which are minimal compared with the overall cost.14 A discount rate of 3.5% was applied for costs and health outcomes as recommended by NICE.22
Parameter distributions for the probabilistic sensitivity analysis
Estimation by subgroups separated demographic heterogeneity from parameter uncertainty, the latter was modelled as a probabilistic sensitivity analysis. The probability distributions used for the input parameters were informed by the sample means and variability. For the parameters of the Weibull survival models, the distributions were multivariate log-normal. To estimate random values from the survival models, the raw coefficients (β0, β1, β2, β3, κ) were assumed to follow a multivariate normal distribution with a correlation structure given by the coefficients from the correlation matrix. Cholesky decomposition of the covariance matrix was performed to simulate the correlated random variates.13 The parameters of the rerevision rate were assumed to follow a beta distribution. The beta distribution was also used to introduce uncertainty in health utilities on the assumption that no value was less than 0, as observed in the data. The gamma distribution was used to model cost uncertainty due to its favourable properties in this context as a positive and skewed distribution.13
Final analyses
The model outcomes were estimated separately by gender and age for each of the three age groups: (1) 60 years or younger, (2) 61–75 years and (3) over 75 years. Mean quality-adjusted life year (QALYs) and costs were calculated for TSA and HA and were presented as incremental cost-effectiveness ratios (ICERs). Monte Carlo simulations were used to address parametric uncertainty by generating 1000 random draws of the assumed statistical distributions for the input parameters. For each one of the three patient subgroups, for a given age and gender, the differential mean costs and QALYs between TSA and HA were calculated. The initial assessment compared these means and established whether one implant dominates the other (if it is less costly and generates more QALYs) or whether it is cost-effective, with incremental costs and incremental QALYs, if the ICER is below the NICE CE threshold established between £20 000 and £30 000 per QALY. The probability of either TSA or HA being cost-effective was calculated for a range of CE thresholds, and the CE acceptability curves (CEAC) were drawn for each patient subgroup. The analyses used to generate the parameter estimates were performed using StataSE V.16 (StataCorp LLC, College Station, Texas). The cost-effectiveness model was constructed in EXCEL V.16.80 (Microsoft Corporation, Redmond, Washington). The Markov models were simulated in Excel.
Patient and public involvement
Patients were involved in the design of the wider body of work comparing TSA and hemiarthroplasty.3 4 The patient and public involvement group at our institution met prior to commencement of the study. This included four surgeons and 32 preoperative and postoperative arthroplasty patients. Further individual discussions were carried out with preoperative shoulder arthroplasty patients.
Results
Input parameter values
HA increased the rate of revision and reoperation for the three age groups, more strongly for revision in younger patients than for over 75-year-olds. The estimated mean EQ-5D-5L utility was higher following primary TSA compared with primary HA (online supplemental table 1). The mean cost of a primary TSA was £6576 compared with £5456 following HA (online supplemental table 14). Other input parameters were taken from the National Tariff Payment System and the literature. The full tables of input parameters are included in online supplemental tables 12–14.
Main findings
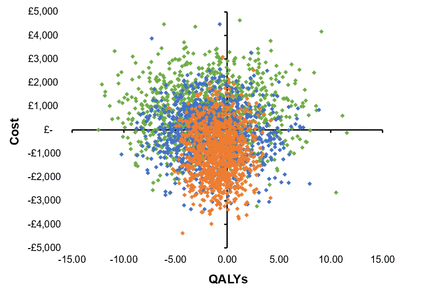
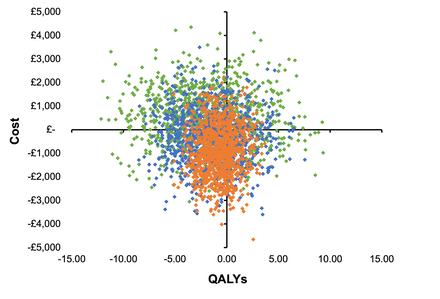
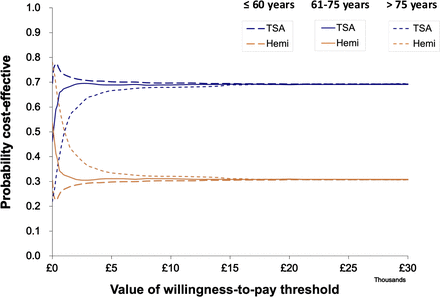
TSA dominated HA in the young cohort, with TSA resulting in mean cost savings of £463 and a 2.0 QALY gain in men, and a saving of £658 and 2.0 QALY gain in women entering the model at age 50 and representing patients aged 60 years and younger (table 1). The cost savings reversed for the older cohort entering at age 80, representing patients over 75, with HA around £966 less costly than TSA in women, and £905 less costly in men but with 0.9 QALYs less than TSA in women and 0.7 in men. For the middle cohort entering the model at age 67 and representing ages 61–75, there was a cost-saving following HA in men and women. TSA resulted in a QALY gain of 1.3 for men and 1.4 for women. The probability of TSA being more cost-effective than HA was constant at around 70% for all willingness-to-pay thresholds considered in decision-making in the UK (£20 000 to £30 000 per QALY). The CE planes for each age group in women and men are shown in figures 2 and 3. The results of the CE analyses are presented for each age cohort. Gender subgroup heterogeneity was indistinguishable from parametric uncertainty in the CE plane; therefore the CEAC are presented for women only (figure 4). The CEAC for men is available in online supplemental figure 2.
Cost-effectiveness plane: female 50—green, female 67—blue, female 80—orange. QALYs, quality-adjusted life years.
Cost-effectiveness plane: male 50—green, male 67—blue, male 80—orange. QALYs, quality-adjusted life years.
Costs, quality-adjusted life years (QALYs) and cost-effectiveness for age and gender subgroups
Cost-effectiveness acceptability curves in women. TSA, total shoulder arthroplasty.
Young cohort ≤60 years
The slightly smaller costs and QALYs for men compared with women were consistent with lower HRs for revision and reoperation in men along with a shorter lifespan: men accumulate less costs and QALYs during the predicted time horizon. The mean ICERs for women and men aged 50 were negative in the North-West area of the CE plane, with incremental costs and decremental QALYs; therefore TSA was dominant. The rates of revision and reoperation were estimated separately for each age within the cohort. There was a decrease in costs and QALYs as age increased (online supplemental figures 4 and 5). The difference in costs between TSA and HA decreased with age reflecting the progressive shortening of life span. In contrast, the difference in QALYs remained similar by age.
The CEACs shown in figure 4 imply that TSA had a higher probability of being cost-saving than HA, even at the willingness-to-pay threshold of £0 (indicating cost-savings), due to the larger hazards of revisions and reoperations for HA than TSA whose costs offset the difference in initial cost. The QALY gain reinforces the probability of TSA being dominant over TA.
Middle cohort 61–75 years
The overall costs for HA were lower than TSA. The cost of TSA was slightly higher following HA in men and women. TSA rendered more QALYs than HA in both men and women, therefore the ICER was positive but on the South-West quadrant of the CE plane. This quadrant is used for disinvestment decisions (withholding the replacement) if the savings are large—at least more than £20 000–£30 000 according to NICE threshold—which was not the case. To show that HA was not cost-effective, the net monetary benefit (NMB) was calculated for women, and it was negative, which showed HA was not cost-effective:
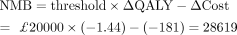
At the willingness-to-pay threshold of £0, there was cross-over; both HA and TSA had the same probability of being cost-saving. The hazards of revision and reoperation were still larger for HA than TSA; however, the middle cohort accumulated less years of costs.
Older cohort over 75 years
The older cohort accumulated the fewest years of costs and QALYs. The hazards of revision and reoperation remained larger for HA than TSA, but the greatest cost saving following HA was shown in this cohort. For both women and men aged 80, HA was less costly than TSA because the HA prosthesis is cheaper and the incremental costs from more reoperations and revisions were negligible. However, the savings did not justify a disinvestment in TSA or replacing TSA by HA. Differential QALYs favour TSA, and the NMB was negative (NMB=20 000*(−0.94)–(−966)=−17 834).
At the willingness-to-pay threshold of £0, HA was more likely to be cost-saving. At a threshold of £1100–£1250 per QALY, there was cross-over in the probability of HA and TSA being cost-effective, and TSA is more cost-effective, with probability up to 68% for men for a threshold over £1250 per QALY.
Discussion
Principal findings
The results showed that cost-effectiveness was likely to be higher following TSA for all age subgroups at a threshold of £20 000–£30 000 per QALY. QALYs were higher for TSA in all age groups. In the young cohort, costs were higher following HA. Despite the lower costs of HA implants and shorter theatre time, this was offset by the additional costs of revision/reoperation. In the older cohort, TSA was more expensive than HA because the higher initial costs were not offset by the lower overall rate of revision and reoperation after TSA during patients’ shorter lifetime. The sensitivity analyses accounted for the uncertainty in the estimates and within each age group, the probability of CE was approximately 0.7 for TSA at current NICE threshold of £20 000–£30 000 per QALY. There is particular interest in the CE of anatomical shoulder replacements in young patients.6 TSA was dominant in patients 60 years and younger at a willingness to pay threshold of £0, primarily due to the large difference in revision rate and longer lifetime of patients in this subgroup, implying TSA is cost saving compared with HA. Postoperative shoulder function may determine whether patients can return to work.23 As the number of shoulder arthroplasties performed each year increases, including in young patients, the loss of productivity due to the additional time required off work should be considered. This further supports the economic arguments for TSA given the superior postoperative shoulder function.
Strengths and limitations
This is the first study to investigate CE in anatomical shoulder replacements using parameter estimates based on national registry data from the UK, and the first to investigate CE in young patients. Age is an important driver of revision rate, and the analysis was split into three age subgroups to better represent subgroup differences in CE across the population. Confounding by indication remained a concern and arthroplasties within each age group were matched on propensity scores calculated from 11 important variables to minimise the risk of baseline differences between the groups. The study was limited to patients with OA and an intact rotator cuff. Revision and reoperation estimates were based on models extrapolated from registry data with a maximum follow-up of 9 years. The sensitivity analysis demonstrated uncertainty in the results. The largest uncertainty was in the utility and cost estimates. The uncertainty in implant survival was smaller.
Modelling assumptions were necessary. Individuals could not undergo revision or reoperation within the first year following the primary procedure, and revision and reoperation could not occur within the same year. We assumed there was no change in utility after the first 6 months, and the annual utility was averaged accordingly. The OSS may continue to improve beyond 6 months after TSA, and the ceiling effect shown in the OSS at 5 years may result in an underestimate of the improvement. The same trajectory of utility following revision surgery was assumed. The utility estimates required transformation of the OSS to the EQ5D. Despite a mapping algorithm based on high-quality data, this introduced additional uncertainty. Revision utilities were estimated by reducing the combined primary utility by 15%. In a prior systematic review, shoulder scores were collected following revision arthroplasty, but only one small study of 15 patients reported OSSs following TSA and none following HA.16 No mapping studies are available to estimate the EQ5D from other shoulder scores.
The cost estimates centred around hospital reimbursement values to improve the generalisability of the results. The cost of theatre time will vary by unit, a range of values are reported, and there is uncertainty among hospital managers.24 The value selected for this work was taken from pooled data from NHS Scotland.21 A median value of implant costs nationally was used to ensure they were generalisable compared with the alternative of relying on procurement costs of a limited number of implants from a single, or small number of hospitals. A single reimbursement code was used for each of the reoperation and revision procedures representing patients with moderate comorbidities. The SD of the cost estimates for reoperation and revision were assumed to be 10% of the cost of the procedure. Postoperative mortality was assumed to be equivalent to age and gender-specific mortality recorded nationally, no evidence could be found to contradict this assumption. Previous work showed there was no difference between the implants at 1 year.4 The national life tables were considered a more accurate predictor of death than summary estimates generated from a relatively small population for this rare outcome. If the rate of death was higher following surgery than in the general population, this may overestimate the CE of both implants.
Comparison to other studies
Prior work comparing the CE of HA and TSA is from North America.7–9 The most recent study by Lapner et al showed TSA was more cost-effective.7 The results were more strongly in favour of TSA than in this study, which may be a product of the difference in North American costs compared with UK costs, and the revision and utility estimates. The utility assumptions for HA were based on patients following proximal humerus fractures, which may underestimate the effect of HA.7 The earlier studies used more limited datasets and showed superiority of TSA to varying degrees.8 9 Given the sensitivity of the models to revision rate shown in this study, the quality of the data used to estimate implant survival is particularly important.
The use of HA has declined; however, it continues to be used, most commonly in younger patients, where there is particular uncertainty about the most appropriate implant.6 Multiple factors are considered when selecting implants for a patient. Prior work has demonstrated a higher revision rate following HA and inferior shoulder scores.3 4 This study showed that TSA was cost-effective in the management of glenohumeral OA, and the superiority of TSA was most clear in the younger cohort, further supporting the use of TSA in patients with OA and an intact rotator cuff.
Data availability statement
Data may be obtained from a third party and are not publicly available. Original data can be requested from the National Joint Registry.
Ethics statements
Patient consent for publication
Ethics approval
The study used pseudo-anonymised data from a national clinical registry. The Health Research Authority guidance confirmed ethical approval was not required.
Acknowledgments
We thank the NJR research committee, and staff at the NJR for facilitating this work. The authors have conformed to the NJR’s standard protocol for data access and publication. The views expressed represent those of the authors and do not necessarily reflect those of the NJR steering committee, research subcommittee, or HQIP.
Footnotes
Contributors AD is the guarantor. AD: conceptualisation, methodology, analysis, writing—original draft. BZ: data curation, methodology, analysis, writing—original draft. SS: conceptualisation, analysis, writing—review and editing. AL: methodology, analysis, supervision, writing—review and editing. MV-B: methodology, analysis, writing—review and editing. AR, PR: conceptualisation, supervision, writing—review and editing.
Funding This work was supported by the British Elbow and Shoulder Society grant number (Ltr014PPG). AD is a Royal College of Surgeons (RCS)/National Joint Registry (NJR) research fellow.
Competing interests PR receives funding for alternative work from Mathys and Orthopaedic Research UK. AR receives funding for alternative work from the NIHR, AO UK&I and DePuy J&J Ltd. AR is a member of the NIHR i4i funding committee. AD, BZ, SS, AL and MV-B have no competing interests.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.