Article Text
Abstract
Introduction In pregnancy, people with obesity or excess adiposity are prone to excess gestational weight gain (GWG) and have the highest risks for multiple maternal morbidities. Epidemiological studies suggest that the lowest incidence of adverse maternal and infant outcomes occurs with GWG lower than current recommendations (<5 kg) and with gestational weight maintenance, resulting in fat mass loss, in those with obesity. Data from randomised clinical trials are needed to evaluate the efficacy of a fat mass loss intervention on pregnancy outcomes. The objective of this proof-of-principle randomised controlled trial is to test the effect of a gestational fat mass loss intervention in pregnant individuals with obesity on changes in weight, fat mass and cardiometabolic disease risk factors.
Methods and analysis In this two-site randomised parallel group, 100 women (30% black; 30% Hispanic) with pre-existing obesity (31.0≤body mass index≤55.0 kg/m2) are randomised to usual care (Provider Directed Group) or usual care plus a fat mass loss intervention with food provision (Weight Maintenance Group). The primary outcomes of the trial (Healthy Mamas/Mamis Saludables) are weight, fat mass (via three-compartment model) and cardiometabolic disease risk factors (ie, blood pressure, lipids, glucose, insulin) from baseline (~13 weeks gestation) to ~35 weeks gestation and at 2 weeks postpartum. Secondary aims evaluate the safety of the fat mass loss intervention during pregnancy and test the hypotheses that compared with usual care, the intervention will have no significant adverse effect on fetal growth, neonatal size, infant body composition and other adverse events. Mediators (eg, eating, activity) and moderators (eg, parity, obesity grade, race/ethnicity) of intervention effects are also examined. Finally, the study will explore the effect of prenatal fat mass loss on reducing the incidence of adverse obstetrical outcomes, including non-elective caesarean delivery, gestational diabetes, hypertension and pre-eclampsia.
Ethics and dissemination The trial has been approved by the Pennington Biomedical Research Center Institutional Review Board, is monitored by an independent data and safety monitoring board and will be conducted in agreement with the Declaration of Helsinki. All results, positive, negative and inconclusive, will be disseminated at national and/or international scientific meetings and in peer-reviewed scientific journals.
Trial registration number NCT04731688.
- Obesity
- Pregnancy
- NUTRITION & DIETETICS
- Pregnant Women
- Randomized Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Healthy Mamas/Mamis Saludables is rooted in extensive prior observational literature suggesting that weight maintenance in pregnant individuals with obesity may reduce adverse pregnancy outcomes and improve the health of mothers and children.
The gestational fat mass loss intervention is more intensive than prior prenatal interventions, adding full food provision, evidence-based calorie restriction and monitoring of weight maintenance goals. Food provision is used to maximise participant adherence, ensure nutritional adequacy and minimise safety concerns.
The same frequency of contact or food provision is not provided to the Provider Directed Group, which could lead to differential retention.
We are not powered to determine intervention effects on the incidence of less frequent but severe obesity-related pregnancy complications. However, recognising this is a proof-of-principle study, we are measuring the incidence of complications to inform a potential future multicentre trial.
Introduction
Pregnancy is a physiologic, anatomic and metabolically dynamic life stage that can be complicated by immediate and long-term effects on maternal health, and pregnant individuals with pre-existing obesity have the highest risk for multiple maternal morbidities.1 2 Half of all pregnant individuals with obesity gain excess weight during pregnancy, which worsens maternal obesity and greatly exacerbates the risk for any maternal and neonatal adverse events.3 4 Social disparities in maternal health outcomes persist, and Hispanic and black individuals experience the highest prevalence of obesity and adverse pregnancy outcomes in the USA.1 5 To incur the lowest risk of adverse maternal and/or fetal outcomes, the National Academy of Medicine (NAM) recommends pregnant individuals with obesity limit total weight gain to 11–20 pounds (5–9 kg) or 0.5 pounds per week (100–200 g per week).6
As summarised in systematic reviews, multicomponent lifestyle interventions with behavioural counselling delivered during pregnancy are effective in reducing gestational weight gain (GWG) and increasing the likelihood for a pregnant person with obesity to achieve guideline attainment.2 4 7 8 A US Preventative Services Task Force systematic review recommended that pregnant individuals receive behavioural interventions in pregnancy to promote GWG within NAM guidelines.8 While this approach implemented at scale would undoubtedly benefit maternal health overall, available research suggests that promoting adherence to NAM guidelines of a 5–9 kg weight gain in people with obesity is likely insufficient to optimise pregnancy outcomes. Several epidemiological studies in individuals with obesity suggest that the lowest incidence of adverse maternal and infant outcomes occurs when GWG is <5 kg and with weight maintenance during pregnancy.9 10
Excess adipose tissue is a major determinant of human health, and GWG is tightly coupled with the gain in adipose tissue mass.11 Preclinical and clinical studies show that maternal fat mass and associated biomarkers (eg, insulin, lipids) and associated adipokines such as leptin are involved in the pathophysiology of adverse pregnancy outcomes such as gestational diabetes mellitus,12 pre-eclampsia13 and infants born large for gestational age.14 In an observational study using doubly labelled water and body composition assessed by the three-compartment model, we observed that fat mass loss was a characteristic of individuals with obesity gaining weight below the NAM recommendations.15 We also observed that the people who gained weight at or below the NAM recommendations consumed fewer calories than their pre-pregnancy energy requirements. Importantly, fetal growth and essential gains in total body water and placenta were preserved. Together, the evidence supports that optimal maternal and child health outcomes may result from a multicomponent behavioural intervention with calorie restriction to promote substantial prenatal fat mass loss in individuals with obesity. However, data from randomised clinical trials are needed to evaluate efficacy.
Healthy Mamas/Mamis Saludables is a two-site, proof-of-principle randomised controlled trial in pregnant individuals with obesity (n=100) to test the effect of gestational fat mass loss on changes in weight, fat mass and cardiometabolic disease risk factors. It will test the primary hypotheses that compared with usual care, a fat mass loss intervention with food provision will result in greater reductions from baseline (~13 weeks gestation) to 35 weeks gestation and at 2 weeks postpartum in the following maternal outcomes: (a) weight; (b) fat mass loss (via three-compartment model) and (c) cardiometabolic disease risk factors (ie, blood pressure, lipids, glucose, insulin). Secondary aims will evaluate the safety of the fat mass loss intervention during pregnancy and will test the hypotheses that compared with usual care, the intervention will have no significant adverse effect on fetal growth, neonatal size, infant body composition and other adverse events. Mediators (eg, eating, activity) and moderators (eg, parity, obesity grade, race/ethnicity) of intervention effects will also be examined. Finally, the study will explore the effect of prenatal fat mass loss on reducing the incidence of adverse obstetrical outcomes, including non-elective caesarean delivery, gestational diabetes, hypertension and pre-eclampsia.
Methods
Trial design
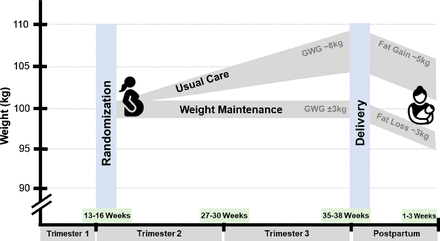
This two-site trial is being conducted at Pennington Biomedical Research Center in Baton Rouge, Louisiana, USA, and California Polytechnic State University in San Luis Obispo, California, USA (Clinicaltrials.gov). One hundred (n=100) pregnant individuals (~30% black; ~30% Hispanic) with pre-existing obesity (31.0≤body mass index (BMI)≤55.0 kg/m2) are randomised to either a Provider Directed Group (usual care) or a Weight Maintenance Group (usual care plus a fat mass loss intervention) (figure 1). Participants are studied from early pregnancy (≤16 weeks gestation) until 2 weeks postpartum. Major assessments occur at baseline (13–16 weeks gestation), 27–29 weeks gestation, 35–37 weeks gestation and 1–2 weeks postpartum. Safety measures are collected from ~week 20 and thereafter at 4–8 week intervals until delivery.
Healthy Mamas/Mamis Saludables clinical trial design. Example weight trajectories for individuals randomised to Usual Care vs Weight Maintenance Groups. GWG, gestational weight gain.
Eligibility criteria
The eligibility criteria were selected to yield a broad group of pregnant individuals with pre-existing obesity who are otherwise healthy.
Inclusion criteria
The trial is open to pregnant (ultrasound confirmed viable singleton gestation, no known fetal anomaly), adult (18–45 years of age) females living with obesity (31.0≤BMI≤55.0 kg/m2) indicated at a screening visit prior to 15 weeks, 4 days gestation to allow for physician clearance prior to enrolment. Enrolment BMI was limited to 31.0 kg/m2 to minimise the likelihood of enrolling an individual with overweight who experienced early pregnancy weight gain and crossed into the obesity category.
Exclusion criteria
Exclusionary factors were identified that could: (1) increase the risks for small for gestation age (SGA) infant (smoking, drug or alcohol use, interpregnancy interval <6 months, conception by artificial reproductive technology, prior SGA infant), (2) negatively impact weight (medication use known to influence body weight, disordered eating, bariatric surgery), (3) require close medical monitoring and additional intervention by providers (prior pre-eclampsia, HIV, active cancer, uncontrolled asthma, haemoglobin A1c (HbA1c) >6.5%, hypertension (systolic blood pressure>160 mm Hg and diastolic blood pressure>110 mm Hg), lupus, chronic renal disease, significant cardiovascular or cardiopulmonary disease, severe anaemia (Hb <8 g/dL and/or hematocrit (Hct) <24%) or (4) compromise successful participation in the intervention (eg, planning to move out of the area in the next 12 months, unwillingness to receive randomisation to either group, unwillingness/inability to eat study foods or to enrol the infant postpartum). Prenatal care providers cleared their patients to participate.
Recruitment and screening activities
Passive recruitment methods include posted flyers, advertisements directed to social media campaigns, email listservs and recruitment services. These are supported by active recruitment methods wherein study staff recruit potential participants at prenatal appointments. Following a brief introduction to the trial, patients are invited to complete a screening questionnaire (via the telephone or internet) to assess their initial eligibility and to collect contact information for follow-up. Participants meeting initial eligibility criteria (ie, age, estimated BMI, gestational age and some medical and medication criteria) are invited for an in-person screening visit. At screening, written informed consent is obtained (online supplemental file 1) and height, weight (BMI), blood pressure, pulse and glycosylated HbA1c are measured. Medical, obstetrical, medication and psychological history are reviewed and the participants understanding of the study and willingness to remain in the study in light of potential obstacles to participation (eg, such as food preferences/allergies, work schedule, family responsibilities, planned travel schedule and driving distance to the clinical centre) discussed. Sample study food items may be provided to the participant to familiarise and consider the ability of a participant to consume the foods included in the Weight Maintenance Group. A medical release is obtained for staff to review the ultrasound and prenatal records to confirm eligibility criteria (ie, gestational age, pregnancy viability, Hb/Hct values and HbA1c). If the eligibility criteria are satisfied, and the participant can be randomised by 16 weeks gestation, then the participant is eligible to continue to baseline assessments and randomisation.
Supplemental material
Randomisation and masking
Participants are randomly assigned within the site (~50 participants per site) and obesity grade to either the Provider Directed Group or Weight Maintenance Group. The randomisation schedule was prepared by the study biostatistician and occurs via Research Electronic Data Capture. Randomisation is performed ≤16 weeks, 0 days gestation, and allocation is concealed from participants until the completion of baseline assessments. Research assessment staff are masked to the group assignment.
Intervention descriptions
Provider Directed Group
Participants in the Provider Directed Group receive what is standard practice by their prenatal care provider during pregnancy. In addition, a brief visit (~20 min) with a study interventionist at randomisation welcomes and bonds participants to the study. Usual care participants receive informational handouts published by the March of Dimes and American College of Obstetricians and Gynecologists on nutrition and activity during pregnancy and general pregnancy topics (eg, limiting caffeine intake, typical frequency of prenatal care visits).
Weight Maintenance Group
Participants in the Weight Maintenance Group receive all aspects of Provider Directed Group plus a multicomponent behavioural intervention with food provision and behavioural coaching to promote healthy eating, activity and the weight maintenance goal of ±3% from randomisation weight (figure 2). The intervention, available in Spanish and English, is rooted in social learning theory16 and based on our prior prenatal interventions.17–19 The intervention is not specific to one particular culture or dietary intake pattern but attempts to provide guidance and resources that may be helpful to individuals from a variety of different cultures, incomes and backgrounds, taking into consideration the balance of caregiver and household responsibilities, cultural norms about social support and recognition of food and activities that are culturally and financially feasible.20 21
Healthy Mamas/Mamis Saludables multicomponent behavioural intervention.
Frequency and mode
Participants in the Weight Maintenance Group receive weekly, 45–60 min, individual, face-to-face counselling sessions in the first 4 weeks of treatment. Thereafter, sessions (~30 min) occur every 2 weeks until delivery; visit frequency may be increased or decreased as needed to promote weight maintenance. The frequency and mode of contact are designed to be intensive enough to promote adherence, provide close supervision and while using rates of clinical contact consistent with clinical practice recommendations.18 22
Weight maintenance goal
Participants receive information on body composition changes that naturally occur during pregnancy in people with obesity and are provided with the overarching goal of maintaining their body weight throughout pregnancy. Participants receive a BodyTrace scale and learn how to monitor and graph their weight. These graphs show the weight maintenance goal with demarcated lines with acceptable variability. If the daily weight is within the goal, the interventionist reinforces adherence. If the weight change is under or over goal, strategies for optimising weight changes are implemented.19
Structured food provision
To support optimal nutrition and steady gestational fat mass loss, structured meal plans with full food provision are provided during pregnancy until 2 weeks postpartum.17–19 Our prior prenatal intervention with partial food provision (replacing two meals) was shown to reduce excess GWG and improve nutritional adequacy.18 Food provision may improve the quality of food stored in participants’ homes, cueing participants to eat these foods, and structure eating in a way that improves portion sizes, eating patterns and knowledge of the energy content and optimal portions of commonly eaten foods. Food provision may simplify the task of following and tracking a prescribed diet and promote adherence.23 24
The food provision plan is individualised and designed to promote 25% calorie restriction during the second trimester followed by isocaloric intake during the third trimester anticipated to result in overall maintenance in body weight (±3%) and a 5% maternal fat mass loss from baseline to delivery. Measured fat mass and fat-free mass from the BodPod at baseline are used to estimate the individual energy requirements and a 25% calorie restriction using our published equation for energy requirements of pregnant individuals with obesity.11
Individually developed meal plans comprise 30% of calories from fat, 15%–20% from protein and 50%–55% from carbohydrates.25 Meal plans include three meals and three snacks for 7 days each week,18 23 24 and the study provides commercially available (ie, Jenny Craig, BistroMD) nutrient-dense foods and high-fibre foods with organic and non-organic options and a combination of frozen and shelf stable foods.18 23 24 Participants supplement these foods and snacks with fruits, vegetables and low-fat dairy. Study foods are provided at no cost and replenished at the convenience of each participant and based on their home storage capabilities. Participants are encouraged to limit sugar-sweetened beverages.
Physical activity
Regular physical activity is encouraged, and participants are recommended to gradually increase moderate to vigorous weekly activity to at least 150 min per week.22 The intervention focuses on increasing ‘lifestyle’ activity or gradual increases in the number of steps walked each day. Participants are encouraged to monitor steps using their personal devices (eg, smartwatch, phone). Each week the goal is to achieve 500 more steps per day (1/4 mile) than the previous week until reaching about 10 000 steps/day.26
Behavioural strategies
Intervention sessions are designed to reinforce adherence to the structured food provision programme, review self-monitoring weight and dietary records, problem-solve barriers and provide additional support. Topics are shown in table 1.
Intervention session topics
Postpartum success
Near the end of the treatment (~35 weeks gestation), participants receive guidance on ways to maintain success after treatment ends. Strategies include continuing the meal plan without food provision,27 28 introducing additional self-selected foods, continually increasing physical activity, being a ‘role model’ and maintaining a healthy home food and exercise environment.
Standardisation of intervention delivery
A treatment manual was developed with a curriculum for each of the topics and counsellors are trained to deliver the intervention under direct supervision. Weekly supervision meetings occur to ensure standardisation. Intervention sessions with participants are audio/video recorded with a subset reviewed and discussed at supervision meetings.
Study visit overview
Participants are enrolled in the study for ~8 months; from the week 13–16 of pregnancy until ~2 weeks postpartum. As shown (table 2), study outcomes are assessed at baseline (13–16 weeks), mid-point (27–29 weeks), late pregnancy (35–37 weeks) and at 2 weeks postpartum. Brief safety measures are collected on approximately a monthly basis throughout the trial, including an additional ultrasound at ~20 weeks gestation. Due to pregnancy complications or to manage study burden, ultrasounds are not required but are encouraged at 25, 31 and 39 weeks gestation. The goal is to collect outcome data at our research facilities, but visits may also occur at the participant’s home, provider office, or elsewhere as needed. Outcome assessment visits are conducted by trained assessors masked to the intervention group assignment.
Schedule of clinical outcome assessments
On the morning of scheduled outcome assessment visits, participants arrive following a fast of at least 6 hours. Participants void, and the collected urine is stored for the total body water assessment and archive storage. Weight is measured, and deuterium is consumed. Additional urine collections are collected at 3 hours, 4 hours and 5 hours after dosing and stored for total body water assessment. Blood pressure, pulse and body composition (BodPod, skinfold thickness and circumferences) are also measured. If the participant is unable to attend the study site for the BodPod measurement, body composition may be measured by a portable bioelectrical impedance device. Adverse events and medication use are recorded, and questionnaires are administered. A fasted blood sample is collected from an antecubital vein for glucose, insulin, lipid panel and to obtain an archived sample for future use (eg, leptin and inflammatory markers). Participants are familiarised with the Automated Self-Administered 24-hour (ASA-24 dietary recall, and an activity monitor is applied. During the subsequent 5–7 days, participants wear the activity monitor and complete the ASA-24 recall on 2 days. The postpartum visit for the mother also includes a body composition assessment via a whole-body dual-energy X-ray absorptiometry (DXA) scan.
Monthly safety visits include measurement of fetal size by ultrasound and record of adverse events and concomitant medications.
Procedures
Maternal anthropometrics and vital signs
Body weight is measured with participants wearing a hospital gown and underwear using the same calibrated scale (Tanita Corp, Arlington Heights, Illinois, USA). Waist and hip circumference are measured using a spring-loaded tape (Gulick II, Country Technology, Gays Mills, Wisconsin, USA). Skinfold thickness is measured at six sites (triceps, biceps, subscapular, iliac crest, mid-calf and mid-thigh) using Harpenden skinfold calipers (Creative Health Products, Ann Arbour, Michigan, USA). Vital signs (blood pressure and heart rate) are measured seated after a 5-min rest. All anthropometric and vital sign measures are recorded two times. If the two trials differ by more than pre-specified value, a third measure is obtained.
Body composition
Maternal body composition is assessed via a three-compartment model in which fat mass is calculated from body weight, body density (BodPod, COSMED USA, Concord, California, USA) and total body water (deuterium dilution).29 Body density is adjusted for trimester-specific changes in thoracic gas volume and fat-free mass hydration.30 Participants receive an oral dose of deuterium (2H2O) as 0.05 g/kg and isotope enrichment is measured in pre-dose and post-dose urine samples at the Mass Spectrometry Core at PBRC.15 Body density (DB) and total body water (TBW) will be used together with body weight to measure fat mass as follows: Fat Mass=(2.118÷DB)−(0.78×TBW÷Body Weight)−1.354.15 At the postpartum visit, DXA is completed to derive a measurement of bone mass which will allow for body composition in pregnancy to also be assessed in a four-compartment model.31
Biological markers of health
HbA1c is measured at screening in capillary blood using a validated point of care system. Serum and/or plasma is measured centrally in the Clinical Chemistry Core at PBRC for glucose, triglycerides, total cholesterol, high density lipoprotein (HDL)-cholesterol and low density lipoprotein (LDL)-cholesterol (DXC600, Beckman Coulter Inc, Brea, California, USA) and insulin (Immulite 2000, Siemens, Broussard, Louisinia, USA). Additional blood samples are stored for future planned assays, such as leptin, adiponectin, high-sensitivity C reactive protein and inflammatory markers (Interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor alpha (TNFα)).
Dietary intake and physical activity
Dietary intake (calories, macronutrient, micronutrient, diet quality) is assessed with the ASA-24 recall through the National Cancer Institute.32 The recall is performed two times by a staff member at the visit or over the telephone; one weekday and one weekend day.33
Physical activity and sedentary behaviour are measured for 5–7 days with activPAL3 micro accelerometer (PAL Technologies, Glasgow, Scotland) secured to the midline of the thigh with waterproof adhesive covering. Participants are asked to wear the device continuously for the assessment period, enabling assessment of time in bed. activPAL data will be analysed centrally using PAL Technologies software. Days with wear time of more than 16 hours and/or days with at least 10 hours of valid awake time will be used in the analysis.34 Outcomes include time spent lying, sitting, standing, stepping, total steps, MET-hours and time spent in different activity intensities (sedentary, light, moderate-vigorous).
Participant-reported outcomes
At baseline, participant-reported outcomes include self-reported pre-pregnancy weight, family medical history, demographics (maternal and paternal), social history (alcohol, tobacco) and food security (United States Department of Agriculture Food Security Module subscale). Outcome assessment questionnaires include: a symptom checklist to measure levels of hunger, fatigue, frequency of constipation and nausea; the Eating Disorders Examination-Questionnaire35 to assess the frequency of unsafe dieting practices; the Edinburgh Postnatal Depression Scale36 to examine levels of depressive symptoms; the Pregnancy Physical Activity Questionnaire37 to assesses time spent in various activity components, including household, occupation, sports and exercise; the Eating Inventory38 to assesses cognitive restraint, disinhibition and hunger; and the Weight Control Strategies Scale39 to measure weight control behavioural and psychological strategies, including dietary choices, self-monitoring, physical activity and psychological coping. Intent to breastfeed at 35 weeks gestation and breastfeeding at 2 weeks postpartum is measured using questions from our prior work40 and originally adapted from the Southampton Women’s Survey and Centers for Disease Control and Prevention Infant Feeding Practices study.41
Intervention adherence and fidelity
Intervention adherence is monitored by tracking attendance at core counselling sessions and self-report of a number of days of consuming provided food. Adherence is also measured through the diet, activity, daily weight and participant-reported outcomes. Intervention fidelity is measured by having 10% of session recordings at each site selected at random and coded for content by independently trained staff.
Chart abstraction
Following delivery and/or pregnancy loss, obstetric outcomes, delivery and neonatal data are collected by review of delivery and newborn medical records abstracted from the electronic medical record or hospital charts.
Fetal ultrasound
Fetal size is measured using conventional two-dimensional ultrasound (biparietal diameter head circumference, transverse diameter and circumference of the abdomen, femur length and humerus length) by certified ultrasonography technicians who are centrally trained and masked to the intervention assignment. Three measurements are recorded, and the mean value used to estimate fetal weight will be calculated using the Hadlock formula.42 For safety surveillance, fetal weight is estimated in real time. Idiopathic fetal growth restriction will be defined as estimated fetal weight <10% of the sex and gestational age norms.43
Infant assessments
Infant weight is obtained using a standard electrical infant scale with the infant undressed. Recumbent infant length is measured using an infantometer, and standard measuring tape is used to measure maximal head and abdominal circumference. Skinfold thickness is measured in duplicate at four sites (triceps, subscapular, iliac crest and thigh). Adiposity is assessed with air displacement plethysmography (PeaPod, Life Measurement, Concord, California, USA)44 and with a three-compartment model using DXA.
Data and safety monitoring
Data across the two sites are collected using standardised case report forms or data upload utilities to a central database at Pennington Biomedical. A Data Safety Monitoring Board (DSMB) comprised of four individuals (physician scientists, clinical researchers and a lay person with lived experience) is convened two times per year and monitors the conduct of the trial. Data on adverse events, recruitment and data completeness are locked at 6-month intervals and used to provide aggregated reports to the DSMB.
Outcomes
The primary outcome of the trial is weight change over time from early (13–16 weeks) to late (35–37 weeks) pregnancy and postpartum (2 weeks). Secondary outcomes for the enrolled pregnant participant are fat mass from early to late pregnancy and postpartum. Other pre-specified outcomes for the enrolled pregnant participant are total body water, fat-free mass, dietary intake, physical activity and maternal cardiometabolic health (eg, blood pressure, lipids, glucose, insulin), perinatal outcomes and fetal growth; and for enrolled infant, neonatal size and body composition.
Sample size and power calculations
Sample size estimates were produced assuming a β=0.8 (power) and α=0.05 (significance) to detect a difference in both body weight and fat mass change between the Provider Directed Group and the Weight Maintenance Group at the end of the intervention. The sample estimates are inflated to allow for an ~8% loss of data while maintaining desired power (β=0.8). The loss to follow-up accounts for participants that may drop out of the study and those that may be required to discontinue due to contraindications, safety alerts or premature delivery of the infant prior to 35–37 weeks. Estimates for the change in total gestational weight and fat mass (35–37 weeks minus baseline) in the Provider Directed group were derived from pregnant individuals with obesity in the MomEE trial.15 The gestational fat mass loss intervention is expected to promote no net weight gain (0 kg) and a 5 kg loss of fat mass. We are sufficiently powered to detect at least a 2.5 kg difference in total GWG (with 92 subjects; 46 per group) and 2.0 kg difference in fat mass gain (with 82 subjects; 41 per group) between the Provider Directed Group and the Weight Maintenance Group at the end of the intervention.
Statistical analysis plan
Statistical analyses will be completed using SAS/STAT software by a study biostatistician (RAB). All analyses will be performed with a significance level of α=0.05. Outcomes will be assessed for normality (where appropriate) with the Shapiro-Wilk test. Non-normally distributed data will be log-transformed; yet, if data are still non-normally distributed following transformation, non-parametric analyses will be conducted on these outcomes Treatment and Obesity Grade will be included in all models. Planned potential covariates will include (1) study site, (2) maternal age, (3) weight at screening, (4) parity, (5) race/ethnicity and (6) gestational diabetes mellitus diagnosis. Intent to treat analysis will be the primary analysis type. Multiple imputation (Markov chain Monte Carlo method, preferred) may be performed if there is a large amount (>10%) of missing data. To decrease the degree of missing data, we will extract the prenatal and delivery record, and, specifically for participants who fail to return to clinic, weight measurements will be acquired from the planned chart abstraction. Mediators (eg, eating, activity) and moderators (eg, parity, obesity grade, race/ethnicity) of intervention effects will also be examined.
Statistical models for the outcomes will use weight, fat mass and cardiometabolic disease risk factors. Results will be expressed as least square means based on the linear mixed effect models for group differences at 35 weeks gestation and 2 weeks postpartum. Secondary outcomes will also use mixed effect linear models to test equivalence between the Provider Directed Group and Weight Maintenance Group for fetal growth, neonatal size and infant body composition at 2 weeks. Maternal appetite, eating disorder, depressive symptoms, reported adverse events, breastfeeding initiation and incidence of SGA will be analysed using a generalised mixed effect model.
Additional exploratory outcomes are dietary intake, physical activity and weight control behaviours, using all available data at the appropriate time points with the addition of appropriate covariates to adjust for potential confounds. Multiple linear regression, logistic regression or repeated measures analysis of variance (ANOVA) will also be used to examine relationships among these variables and associations with weight changes; we will follow approaches outlined by Kraemer et al,45 to explore potential mediators and moderators of treatment outcome. As we also present exploratory aims, results for frequencies of adverse obstetrical outcomes by treatment conditions will be expressed as contingency tables with incidence rate. Generalised linear mixed model may also be used if covariates have a significant impact on model fit.
Patient and public involvement
The intervention was developed using acceptability ratings among obstetricians and individuals of childbearing age with obesity. Obstetricians reported that they would feel ‘moderately’ to ‘extremely’ comfortable if pregnant patients with obesity underwent ‘a lifestyle programme that promoted modest (5%) fat loss during the second trimester of pregnancy followed by weight maintenance during the third’. Importantly, most reported they were willing to refer patients to our proposed lifestyle intervention. Among individuals of childbearing age with obesity, there was a high acceptability of gestational weight loss and/or gestational weight maintenance to promote pregnancy health.
Discussion
Observational data suggest that pregnant people with obesity may have the best pregnancy outcomes with gestational weight maintenance, but clinical trials are lacking. Without such trials, the potential benefits or harms of gestational weight maintenance and fat mass loss during pregnancy are unknown, there can be no practical recommendations for individuals with obesity to continually manage their obesity in pregnancy.46–48 An estimated 1.1 million individuals in the USA enter pregnancy having pre-existing obesity each year49 and are at high risk of experiencing life-long health problems for themselves and their children. If intentional fat mass loss during pregnancy is shown to be safe and effective, the prenatal period would remain the most opportune time to intervene, given that nearly all individuals intersect with healthcare delivery during this life stage and with frequent monitoring.
The trial includes two sites with a goal of enrolling a diverse sample of pregnant individuals (~30% Hispanic and~30% African American). The only other trial to explore GWG throughout pregnancy50 51 used a lower intensity intervention, had minimal racial/ethnic and socioeconomic diversity and lacked measures of cardiometabolic health. The proposed study addresses these limitations by intensifying the intervention on an individual level with the use of food provision based on published estimates of energy requirements for pregnant individuals with obesity, inclusion of accurate body composition and cardiometabolic health measures and enrolment of a diverse sample at greatest risk of obesity-related disease.52
The intervention’s use of a calorie prescription derived from highly rigorous research on maternal body composition and energy expenditure15 and the provision of full meal replacements 7 days per week is novel in pregnancy research. Outside of pregnancy, food provision, with portion sizes and nutritional content being controlled, has been shown to lead to more weight loss than either self-directed dieting or structured behavioural weight management programmes without food provision and in diverse populations.24 53–56 Commercially provided food provision programmes with physical activity and behavioural strategies have shown 2–3 times greater weight losses.23 53–58 We previously tested the efficacy of a behavioural programme with partial meal replacement during pregnancy and found that it effectively reduced excess GWG and improved nutritional adequacy in individuals with obesity.18 Food-as-medicine initiatives59 provide access to optimal nutrition, reduce adverse health risk factors and improve health outcomes, which may subsequently lower the overall cost of current and future healthcare. If programmes, such as Healthy Mamas/Mamis Saludables, are successful, future implementation research would be warranted, and food provision and prescriptive diets as part of comprehensive lifestyle interventions may become a formal, consistent component of pregnancy and postpartum care to enhance short- and long-term health in pregnant people with obesity.
The trial design has many strengths. Healthy Mamas/Mamis Saludables is rooted in extensive prior observational literature including hundreds of thousands of pregnancies that have suggested that weight maintenance in pregnant women with obesity may reduce several adverse outcomes and improve the health of mothers and children. We are incorporating a highly innovative gestational fat mass loss intervention, which was informed by our own pilot data.15 We anticipate a large effect size of the trial. Based on our pilot data, we anticipate 67% of individuals in the usual care group will gain weight in excess of NAM guidelines (>9 kg). However, we conservatively powered the current trial assuming that 50% of those in the usual care group would gain in excess, allowing for an inflated sample size to test the feasibility and acceptability of a fat mass loss intervention of a prolonged intensive behavioural intervention prescribing a 25% calorie restriction. We are also using state-of-the-art outcomes and procedures across two highly skilled sites known for their work in pregnancy interventions and recruitment of diverse populations.
It is possible that the intervention will not produce gestational weight maintenance. However, we have designed the intervention to expand or contract in intensity, as needed, to facilitate intervention goal attainment. Similarly, we will not be providing the same frequency of contact or food provision to the Provider Directed Group, which could lead to differential retention rates. However, we have had extremely high retention rates in our non-intervention control groups in prior completed and current ongoing studies. In our prior trial, we provided evidence that a partial meal replacement programme reduced GWG by ~2 kg. Our current intervention builds on and intensifies this work by adding full food provision, evidence-based calorie restriction and the provision and monitoring of weight maintenance goals. Collectively, these elements are anticipated to produce the strongest prenatal weight management intervention effect documented to date.
Ethics statements
Patient consent for publication
References
Footnotes
X @JohnApolzan
Contributors LMR and SP are the principal investigators of the trial, wrote the initial draft of the manuscript. LR serves as the guarantor. JWA, RAB, ADA, MSD, ES, EWF, HEC, JRS, MK, ABC, AMV, DSH, EY and SKK are involved with the study design and/or conduct of the trial and provided feedback and approved the manuscript.
Funding This research is funded by the National Institutes of Health grant 5R01DK124806 (Redman and Phelan), T32DK064584 (Cabre), F32HD10822 (Flanagan) and with support of Cores within the Pennington/Louisiana Nutrition Obesity Research Center (P30DK072476) and Louisiana Clinical and Translational Research Center (U54 GM104940).
Competing interests Unrelated to this work, SP has a grant from WeightWatchers. All other authors have no competing interest to declare.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Author note Throughout this manuscript, the authors refer to pregnant individuals as ‘mothers’ and use the verbiage ‘maternal’ . The authors acknowledge that gender non-conforming people and those that identify as male assume such roles. The wording in the manuscript was chosen for ease of reading.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.