Article Text
Abstract
Introduction Internationally, breast cancer is the second most diagnosed cancer with approximately 2.3 million people diagnosed each year. 40% will require a mastectomy which has an average length of hospital stay of 1–2 days. Enhanced Recovery After Surgery (ERAS) guidelines include the following patient-managed recommendations: early mobilisation, early eating and drinking, opioid minimisation and physiotherapy exercises. Low adherence rates to these recommendations suggest that patients need support to do these things. A digital health intervention (DHI) may provide an effective, cost-effective and scalable solution. This pilot trial aims to assess the feasibility of conducting a trial of RecoverEsupport and the acceptability of the RecoverEsupport intervention to support patients to recover from breast cancer surgery.
Methods and analysis Participants will be recruited from the perioperative clinic and breast surgery units at a major cancer hospital in New South Wales, Australia and randomised (1:1) to receive (1) control: usual care or (2) intervention: usual care plus RecoverEsupport. The DHI incorporates the following evidence-based behaviour change strategies: education, self-monitoring and feedback and prompts and cues. The primary trial aims are to assess the feasibility of the trial and the acceptability of the RecoverEsupport intervention. The secondary aims are to assess preliminary efficacy and cost-effectiveness regarding the length of hospital stay. Data regarding patient behaviours related to patient-managed ERAS recommendations, Quality of Life, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), Quality of Recovery (QOR-15), Anxiety (Hospital Anxiety and Depression Scale), hospital readmissions, emergency department presentations and health service utilisation postdischarge will also be collected.
Ethics and dissemination This study has been approved by the Human Research Ethics Committees of the Hunter New England Local Health District (2022/ETH02010), the University of Newcastle (H-2023–0298) and the Calvary Mater Newcastle (2022/STE03757). Trial outcomes will be disseminated via peer-reviewed publications and conference presentations.
Trial registration number Australian New Zealand Clinical Trials Registry (ANZCTR) ACTRN12624000417583.
- Behaviour
- Digital Technology
- Breast tumours
- eHealth
- Patient-Centred Care
- Breast surgery
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The RecoverEsupport trial will address a clinician-identified evidence gap by providing a digital health solution to support patients with breast cancer in enhancing their recovery from surgery.
This intervention development has been informed by and co-designed in collaboration with clinicians and consumers.
The digital health intervention is designed to be accessible on any internet-connected device.
This trial uses a randomised controlled trial (RCT) design and uses routinely collected length of stay data from medical records.
This pilot RCT has been designed to inform decisions about proceeding to a fully powered RCT and while it will provide an initial estimate of effect size it has not been powered to detect between group differences.
Introduction
Background and rationale
Worldwide, breast cancer is the second most diagnosed cancer with around 2.3 million people diagnosed each year and over 685 000 deaths annually.1 Globally, expenditure on breast cancer is estimated to exceed US$2 trillion by 2050.2 Most women with breast cancer will undergo surgery,3 with approximately 40% having a mastectomy4 requiring a hospital stay averaging between 1–2 days.5 Around 50% of those women will choose to have a breast reconstruction following a mastectomy4 with implant-based reconstructions requiring an additional average length of stay of 1–2 days.6 Complications occur in around 10% of breast cancer surgical patients3 and patients need to be physically and mentally recovered from their surgery, to return to their daily lives, and to prepare for the next phase of their treatment regime, usually chemotherapy and/or radiation therapy.7 As health services transition towards more patient-centred care models, it is essential to consider the role patients can play in their recovery. As such, best-practice and internationally endorsed Enhanced Recovery After Surgery (ERAS) guidelines include specific patient-managed recommendations in addition to clinician-managed recommendations, to optimise patient recovery outcomes. A 2019 systematic review of ERAS pathways in breast reconstruction suggests that the implementation of ERAS guidelines for patients reduces the length of hospital stay without increasing postoperative complications, decreases opioid use and can improve quality of life (QoL).8 These findings were also supported by a 2023 retrospective study which investigated outcomes for 92 patients having a single mastectomy. Findings for the 32 patients managed in the ERAS group suggested that implementing ERAS pathways for mastectomy patients (without immediate reconstruction) is associated with a shorter length of hospital stay and a reduction in postoperative complications.9 The patient-managed recommendations include:
Pre-surgery: preadmission optimisation (smoking cessation, achieving a healthy weight, being physically active and alcohol reduction/cessation and provision of information and education).
Post-surgery: early mobilisation, rapid resumption of oral feeding and drinking, opioid minimisation and physiotherapy exercises.
Post-discharge: home support (supportive care for the management of drains and wounds) and physiotherapy exercises.10
Non-compliance with ERAS guidelines including early resumption of feeding and postoperative mobilisation, has been associated with higher rates of postoperative complications.11 Although reports vary, poor adherence to ERAS recommendations is well documented and to date, no studies of adherence to the patient-led ERAS guidelines within patients with breast cancer could be identified. However, a prospective study of 1391 patients undergoing colon surgery reported non-compliance with early feeding and mobilisation in up to 30% of patients which was associated with higher rates of postoperative morbidity.11
Resource constraints can impede patient adherence to ERAS recommendations through limited communication and collaboration between staff and patients, resistance to change from patients and staff and limited patient education preparing them to take an active role in their recovery.12 Digital health interventions (DHI) may provide a way of addressing these barriers. DHIs have been shown to be effective in producing health behaviour change in patients with cancer13 and offer advantages over more traditional forms of support in that they can be tailored, are highly scalable, are cost-effective, and can support patients within and beyond the hospital setting.14 Evidence also suggests that DHIs have been effective in changing behaviours and managing symptoms such as pain and anxiety in patients with cancer.7 As such, a DHI may support patients to adhere to the patient-led ERAS recommendations. Given the high prevalence of breast cancer surgery and the limited evidence regarding effective, cost-effective and scalable interventions to enhance patients’ recovery from surgery, a DHI has been developed to increase adherence to the patient-led ERAS recommendations.
While previous literature shows that adhering to ERAS recommendations improves clinical outcomes,8 10 there is minimal research indicating how patients can be best supported to adhere to these ERAS recommendations. To date, there has been no randomised controlled trial (RCT) evaluating a behavioural intervention to support patients with breast cancer adhering to the comprehensive set of patient-managed ERAS recommendations across the perioperative period. Prior to conducting a fully powered RCT to evaluate the effectiveness of this intervention, this pilot RCT will be used to determine the acceptability of the DHI and the feasibility of components of the research design.
Methods and analysis
Objectives
The primary aim of the trial is to assess the acceptability of the RecoverEsupport intervention to support patients in recovering from breast cancer surgery (mastectomy with or without reconstruction or reconstruction following a previous mastectomy) and to assess the feasibility of conducting a fully powered RCT. The secondary trial aims are to assess preliminary efficacy and cost-effectiveness, specifically looking at the estimate of the variability of the RecoverEsupport treatment effect on the length of hospital stay (assessed via medical records). Other outcomes to be assessed include patient behaviours related to patient-managed ERAS recommendations, quality of recovery, anxiety, QoL, health service utilisation post-discharge (assessed at 90 days postoperatively), hospital readmissions and emergency department presentations. Participants will also have the option to participate in an optional interview to identify relevant themes relating to their experience of RecoverEsupport.
Trial design
The trial design is a two-armed pilot RCT with participants randomly allocated to receive (1) control: usual perioperative care; or (2) intervention: usual care plus access to RecoverEsupport, an online programme to support patients recovering from their surgery. Outcome measures will be assessed in-hospital postsurgery and at 1 and 3 months post-surgery.
This paper outlines the trial protocol based on the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) recommendations. A completed SPIRIT checklist is available (see online supplemental file). The trial was prospectively registered with the Australian New Zealand Clinical Trial Registry ACTRN12624000417583 and any future modifications will be added as approved.
Supplemental material
Study setting
The study will take place in the perioperative clinic and breast surgery units at a major cancer hospital in a regional metropolitan area15of New South Wales, Australia. The trial will run from July 2024 and will recruit for approximately 18 months.
Eligibility criteria
Patients are eligible if they are female, aged over 18 years, with either a planned mastectomy for breast cancer (with or without a reconstruction), or a delayed implant-based reconstruction following a previous mastectomy, are English speaking and free from cognitive and emotional impairment (if the recruiting nurse thinks receiving the information about the study at any time will cause the patient undue distress/anxiety), and have internet access and access to an email address. Patients unable to provide independent informed consent; patients having autologous reconstructions (deep inferior epigastric perforator flaps, transverse rectus abdominus myocutaneous flaps, latissimus doris flaps); those who have previously participated in this study; and those who need emergency surgery will be excluded.
Recruitment
Patients will be invited from the public and private perioperative clinics of the surgeons. Potential participants will be made aware of the study via a flyer. Hospital staff will identify eligible patients scheduled for surgery and will approach them via telephone or in-person (if attending the public clinic) using a recruitment script that was pre-approved by the ethics committee to ensure compliance with ethical standards. A recruitment pack (containing an invitation letter, participant information statement and a link to the online consent form (online supplemental file 2) and a reminder 5 days later will be emailed to all interested patients. Hospital staff will also seek patient permission for a member of the research team to contact them to answer any questions and confirm their interest in participating. Where provided, the age and reasons of non-consenting patients will be recorded to examine consent bias.
Supplemental material
Ethical considerations
This study has been approved by the Human Research Ethics Committees of the Hunter New England Local Health District (2022/ETH02010) and the University of Newcastle (H-2023-0298) and the Calvary Mater Newcastle (2022/STE03757) and was prospectively registered.16 Each invited patient will receive a patient information sheet inviting them to participate in the pilot study and optional qualitative component, assuring them that their participation is entirely voluntary and that any information they provide will remain confidential. No incentive will be offered to patients to participate. All project staff are bound by confidentiality agreements. Collected data will be stored in a non-identifiable format.
Randomisation
At the completion of the baseline survey or if participants are within 5 days of surgery and have not yet completed the baseline survey, participants will be randomised via a randomisation module within REDCap17 in a 1:1 ratio in block sizes varying randomly from 4 to 6 to either the intervention or control group. Given the nature of this behavioural intervention, blinding participants to their group allocation will not be possible. However, neither participants nor clinicians will be aware of participants allocation at study enrolment. In addition, intervention participants will be placed into a subgroup analysis comparing two different schedules for receiving postdischarge exercise information: Group A—will have the option of setting their own schedule to receive postdischarge exercise reminders and Group B—will receive reminders according to a preset schedule to explore the impact of different schedules. In order to avoid a potential imbalance in the subgroup due to minimum block sizes and to keep the main study outcomes independent, the intervention subgroups were established via a different method. Inside REDCap, a pseudorandom number generator would allocate each intervention participant a value between 0 (inclusive) and 1 (exclusive). Intervention participants with a value≥0.5 were allocated to Group A and intervention participants with a number <0.5 were allocated to Group B.
Usual care
All patients will attend a presurgical and/or perioperative appointment (either face-to-face or via telephone) where they may meet with a breast care nurse and the surgeon and/or anaesthetist. The interventions provided postoperatively in the hospital under usual care include standard pain management with the use of medications, standard wound care, physiotherapy exercises and discharge planning and education. All patients who require drains will also receive standard postoperative information and care from a breast care nurse. Patients discharged with drains still in place will receive ongoing care and communication from a community nurse or hospital in the home until the drains are removed.
Intervention development
The intervention (figure 1) was developed based on an existing intervention for bowel cancer surgical patients18 and has been adapted for breast cancer surgical patients using iterative feedback from clinicians, consumers and previous breast cancer surgical patients.
RecoverEsupport homepage.
Theoretical basis for the intervention
The COM-B (Capability, Opportunity, Motivation- Behaviour) framework has been used as the theoretical basis for intervention development, a behavioural theory used in both psychology and healthcare to help address barriers related to the capability, opportunity and motivation to engage in specific behaviours.19 Table 1 outlines the behaviour change techniques used within the intervention and has been based on evidence-based behaviour change techniques taxonomy.20
Behaviour change strategies used in the RecoverEsupport intervention
Intervention description: RecoverEsupport
Access to the online programme
Immediately following randomisation, a personalised alert (SMS/email) and link to the ‘prescription’ letter from a surgeon, will be sent to all intervention participants. The letter will introduce the online programme and will outline the potential benefits of using the programme, provide instructions for access and include contact details of the research team. All participants will be sent two reminders to use the programme, approximately 3 and 7 days later. The online programme will be available for the participant to access on-demand until 3 months postsurgery. Participants will be encouraged to bring a digital device (laptop/smartphone/tablet) to the hospital to access the programme during their stay. Paper copies of the daily checklists will be provided in case participants cannot access the online programme during their hospital admission.
The online programme
The intervention consists of the following components: information, videos, quiz questions (with real-time feedback provided for incorrect responses), daily checklists (monitoring and feedback) and SMS/email alerts and reminders to improve patient knowledge and motivation to adhere to certain behaviours. The content will cover:
Pre-operative support (preparation for surgery including recommended physical activity guidelines, alcohol and smoking reduction strategies, admission procedures),
Post-operative support (eg, early mobilisation, early eating and drinking, pain relief, exercises and self-care/psychosocial care (the 5 ‘Recover-Es’) as well as discharge procedures) and
Post-discharge support (eg, physiotherapy exercises, wound and drain management and managing follow-up appointments). Strategies to self-manage physical and psychological issues will also be included.
The programme content is based on best practice guidelines,10 evidence from the literature and feedback from the clinical advisory group members, consumers and patients.
Online programme delivery
The online programme will be delivered via REDCap. REDCap allows participants to access multimedia content via their own devices (computer/smartphone/tablet). The programme will be used to: provide participants with information; collect information from participants; transfer information via the internet; and store information in a secure, central data collection repository.
Ongoing participant access
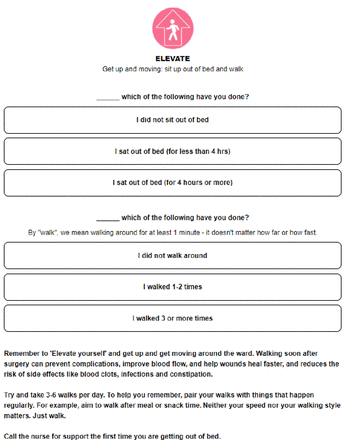
Participants will have access to the online programme presurgery and postsurgery, including during their hospital admission and up until 3 months post-surgery. They will receive a printed reminder in their hospital room to access the programme. Intervention participants will be asked to complete a brief online checklist (Figure 2) each day (daily checklists) to assess the extent to which they are following recommended self-management strategies to aid recovery (eg, early mobilisation following surgery). Responses that indicate non-adherence to recommendations will be flagged with the breast cancer nurses who will follow up as required.
Daily checklist example.
Outcomes
Participants will complete surveys at four time points: baseline, post-surgery (in hospital) and 1 and 3 months post-surgery. The baseline survey is sent following consent and if not completed, reminders will be sent approximately 3 and 6 days after the initial invitation. The same reminder procedure used at baseline will also be used for all subsequent surveys. The post-surgery survey is a brief online assessment that will be completed on Day 2 post-surgery. Printed copies will be made available for participants who cannot (or do not) access the in-hospital survey online. Participants will be sent SMS/email reminders to complete each survey within the next 48 hours (the next 24 hours for the in-hospital survey).
Primary trial outcomes
Feasibility of the RecoverEsupport intervention will be assessed based on whether the following prespecified targets are met:
Participant recruitment (Target: n≥70 participants consent to the study).
Retention rate (Target: ≥85% remain in the study at 1-month follow-up (ie, have not withdrawn).
Data collection (Target: ≥85% of participants from the study have length of stay data collected).
Adverse events assessed at 1-month follow-up (Target: no adverse events classified as grade 3 or above, based on items adapted from the Common Terminology Criteria for Adverse Events (V.5.0) including falls, muscle pain/discomfort and anxiety). This is a widely used measure for the reporting of adverse events whereby events are graded on a scale from 1 to 5 with higher grades indicating more severe adverse events.21
Acceptability of the RecoverEsupport intervention will be assessed among intervention participants only and will be based on the following:
Intervention usability (Target: average score on the System Usability Scale>68, ie, ‘Okay’ or higher), measured at 1-month postsurgery. The System Usability Scale is a widely used standardised, brief 10-item scale to assess intervention usability that has good validity and reliability.22 Total scores range from 0 to 100 with scores>68 classed as satisfactory.
Intervention engagement: use of RecoverEsupport, measured at 1 month and 3 months postsurgery. Use of the online programme will be monitored through analytics automatically recorded by REDCap. (Target: ≥75% of participants logged onto RecoverEsupport at least once).
Intervention component acceptability: a series of questions using Likert scale response options will assess the acceptability of characteristics of the intervention components, measured at 1 month postsurgery. Questions (Likert scale) will assess the ease of use, relevance and quality of the support and information accessed. Participants will also be asked if they would recommend the programme to other people having surgery. (Target: ≥75% of participants would recommend RecoverEsupport).
Secondary outcomes
Preliminary efficacy
Length of stay will be calculated as the date of discharge less the date of admission, based on information extracted from the patients’ medical records.
Patient ERAS knowledge and behaviours (measured postoperatively in-hospital)
Patient behaviours related to patient-managed ERAS recommendations (preadmission behaviours and mobilisation, oral diet, fluid intake, opioid minimisation and physio exercises) will be assessed via questions developed specifically for the study.
Quality of Recovery (in-hospital, 1 month and 3 months follow-up)
The QoR-15 is a validated and reliable tool that assesses the early post-operative health status of surgical patients.23 The sum of all scores ranges from 0 to 150 with higher scores indicating a better quality of recovery.23
Anxiety (baseline, 1 month and 3 months follow up)
Assessed via the Hospital Anxiety and Depression Scale (HADS), a valid and reliable assessment that measures anxiety for patients in clinical settings.24 This is a widely used tool which has two scales, one for anxiety and one for depression. The Anxiety scale has 7 items which are summed up to form a score out of 21: 0–7 = normal, 8–10 borderline abnormal, 11–21 abnormal.25
QoL (baseline, 1 month and 3 months follow-up)
Assessed using the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30 V.3.)26 The QLQ-C30 is a 30-item cancer-specific instrument that measures 5 functioning domains (physical, role, cognitive, emotional and social), 9 symptom scales (fatigue, nausea/vomiting, pain, dyspnoea, sleep disturbance, appetite loss, constipation, diarrhoea and financial impact), as well as global QoL and provides a summary score.26 Scores for each scale range from 0 to 100. Higher scores for global health status indicate a high level of functioning and a high QoL.27 The clinical validity of the scale is high and test–retest reliability is psychometrically credible.28
Health service utilisation
Hospital readmissions, emergency department visits and health service utilisation postdischarge will be assessed using an adapted version of the Client Service Receipt Inventory at 3 months postsurgery.
Cost data was collected throughout the duration of the intervention based on detailed project management records, assessed at the conclusion of the study.
Nested trial outcomes
The average number of completed exercise log entries (as recorded in REDcap) and the average number of completed exercise sessions (as reported by the participant in the 1-month follow-up) will be collected.
Other data collected
Demographic characteristics (baseline)
Participant demographics including age, gender, education, country of birth, home postcode, language spoken at home, marital and employment status, health risk behaviours, internet use and surgical history will be obtained from the medical record, consent form and baseline survey.
Medical record data (until 3 months postsurgery)
Treatments received and length of stay data will be collected from a medical record audit.
Analytics
Use of the online programme will be automatically recorded by REDCap.
Qualitative Interview
At the end of the study, a research assistant will contact participants who indicated that they were willing to take part in an interview about their experience with RecoverEsupport. This is to ensure that data is collected about participants’ broad experiences using the intervention, to ensure that key aspects of their experience are not missed and to supplement the quantitative outcomes reported. This qualitative research component will be conducted using semistructured interviews to establish the acceptability of RecoverEsupport. The interview will be based on a predetermined discussion guide and will be conducted via phone call and recorded.
The data collection schedule is outlined in table 2.
Data collection schedule
Sample size
Given the nature of the trial, the sample size was determined based on the number of participants needed to assess the feasibility of the study protocol, obtain measures of study acceptability to inform the decision-making process to proceed to a larger RCT29 and provide relevant information on adverse events.30 A sample of 35 participants per experimental arm (approximately 70 in total) is the enrolment target for this study with similar studies reporting sample sizes between 30 and 36.31 Based on patient volume at the hospital, it is anticipated that recruitment will take 18 months. This study is not powered to detect significant differences in outcomes but to perform an exploratory analysis only. It is estimated that 10 participants will be required to identify relevant themes for the optional qualitative interviews.
Analysis
Table 3 details the criteria for advancing to a larger trial. The criteria has been established based on recommendations for progression criteria for pilot trials and feasibility studies32 33 and will be used by the study team to evaluate whether a larger trial is warranted and to inform any required changes to the protocol.
Progression criteria for advancing to a larger trial
Demographic and treatment/disease characteristics: Baseline characteristics of the treatment groups will be described using descriptive statistics.
Secondary outcomes
To explore the preliminary efficacy of the intervention to inform a fully powered RCT, between group differences in length of stay will be examined. Analyses will be conducted on an intention-to-treat basis with the Bayesian framework using non-informative priors. A per-protocol analysis will also be conducted and exploratory subgroup analyses will be conducted to explore the influence of participant age. Other secondary outcomes will be modelled using regression with adjustment for baseline values and distribution where appropriate.
Nested trial
Among participants randomised to receive the RecoverEsupport intervention, regression analysis will be conducted to investigate the between-group difference (A vs B) in the average number of completed exercise log entries and the average number of completed exercise sessions.
Economic analysis
Subject to the assessment of feasibility, acceptability and preliminary efficacy, a trial-based economic evaluation involving costing, cost-consequence and cost-effectiveness analysis will be conducted. The analysis will compare the RecoverEsupport intervention against the control group (usual care) from a health service perspective. Resource use will be identified and measured for the intervention implementation. It will be assumed that the resource use in the usual care group is zero. The incremental cost-effectiveness ratio will be calculated as the between-group difference in mean total implementation cost divided by the observed between group difference in the length of hospital stay. Sensitivity and scenario analysis will be undertaken to test the impact of changing key design features of the intervention.
Qualitative interviews
Participant interviews will be transcribed and a thematic analysis conducted to identify emerging themes.
Implications
This study will gather evidence through the rigorous testing of the trial protocol, randomisation and recruitment processes, and the intervention itself, to identify any required amendments to inform the design of a future fully powered RCT. This will contribute to the evidence base for strategies to support patient self-management during the perioperative period for patients with breast cancer. The behavioural strategies used in the RecoverEsupport DHI are grounded in theory and experimental literature and are intended to support patients to take an active role in managing their preparation and recovery from surgery.
The intervention has been designed to maximise its potential for adoption; and because key components of the intervention are online, it can be centrally managed and customised for different health services at relatively low cost. The use of digital technology has the potential to make cost-efficient use of scarce healthcare resources while providing personalised information and support for surgical patients. This technology can be readily integrated into routine care. While this study focuses on breast cancer surgery, the principles underpinning the intervention can be readily adapted to other types of surgery (both cancer and non-cancer). A limitation of this research is that the intervention has only been developed for English speakers. Should the intervention be established as feasible, acceptable and efficacious, it could be adapted for other patient groups including non-English speaking and hard-to-reach populations. With surgery being one of the most common treatment options for breast cancer, with between 80% and 96% of patients with breast cancer undergoing surgery,34 35 this intervention represents a potential opportunity to improve patient recovery outcomes while improving the efficiency of care.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to acknowledge the Cancer Institute NSW, University of Newcastle, Calvary Mater Newcastle, Clinicians, consumers from Cancer Voices Australia, as well as Lucy Leigh for her statistical advice and Patrick Skippen for his REDCap expertise
References
Footnotes
X @mitchjduncan
Contributors ES is the guarantor and was responsible for drafting the work and giving final approval to the version submitted. ES led the development of this manuscript. RW secured the funding. RW, ES, AZ, OM, PVdS, SRS, SR, RC, HM, LR and AP-P developed and refined the intervention strategies. RW, ES, AZ, MJD and SRS determined the measures to be used and RW, ES, MJD, PR and CO determined the analysis to be conducted. ES, RW, AZ, MJD, OM, PVdS, SRS, SR, RC, HM, LR, AP-P, PR and CO contributed to the research design and trial methodology and read and approved the final version of this manuscript.
Funding This work is being supported by the Cancer Institute NSW Australia (Grant number:2021/ECF1355) and the University of Newcastle. RW also receives salary support from this grant. ES receives a PhD stipend funded through this grant. MJD is supported in part by the Commonwealth of Australia 2022 Effective Treatments and Therapies Grant (MRF2023434)
Competing interests None declared.
Patient and public involvement Two consumers from Cancer Voices were given an early prototype to test and provided feedback which informed the subsequent versions of the intervention. Furthermore, a qualitative study with 10 former breast cancer surgical patients was conducted and feedback from their testing also influenced the final version of the intervention
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.