Article Text
Abstract
Introduction Tuberculosis (TB) continues to be a significant public health issue, particularly in low-income and middle-income countries. Globally, the End TB Strategy targets an 80% reduction in TB incidence by 2030. Despite this strategy, there remains limited evidence on the incidence of TB among HIV-infected children after the test-and-treat strategies in Amhara Region. Hence, this study aimed to assess the incidence of TB and its predictors among children on antiretroviral therapy (ART) in Amhara Region, which is the second largest region in Ethiopia and located in the northwestern, northeastern and north-central parts of Ethiopia.
Methods A multicentre institution-based retrospective follow-up study was conducted on 421 HIV-infected children receiving ART from July 2014 to March 2022. The study participants were selected using simple random technique. National antiretroviral intake and follow-up forms were used to gather data through the KoBo Toolbox. Stata V.17 was used for data analysis. The Kaplan-Meier curve was applied to estimate failure time, and the log-rank test was employed to compare groups of predictors. To identify TB predictors, Gompertz regression models, both bivariable and multivariable, were constructed. Ultimately, a 95% CI adjusted HR (AHR) was calculated, and variables with a p value less than 0.05 were considered statistically significant.
Results A total of 421 children with a record completeness rate of 97.9% were analysed in the study. The TB incidence rate in children on ART was 2.16 (95% CI 1.52, 3.05) per 100 child-year observations. Anaemia at baseline (AHR: 3.83; 95% CI 1.46, 10.04), never taking TB preventive treatment (TPT) (AHR: 3.78; 95% CI 1.44, 9.94), wasting (AHR: 2.53; 95% CI 1.19, 5.38) and not initiating ART within 7 days (AHR: 2.35; 95% CI 1.15, 4.78) were significant predictors of TB in children.
Conclusion The incidence of TB in children on ART was relatively high. HIV-positive children presenting with anaemia, those who never took TPT, wasted children and those with late initiation of ART were prone to the occurrence of TB. Therefore, prioritising anaemia treatment, TB preventive therapy, nutritional counselling and timely initiation of ART are essential to curb the TB burden.
- EPIDEMIOLOGY
- PUBLIC HEALTH
- Community child health
- INFECTIOUS DISEASES
- Hospitalization
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
As a strength, this study is multicentre, covering a wide ranges in geographical areas of specialised comprehensive hospitals.
Due to the nature of a retrospective follow-up study, factors such as housing characteristics, the environment and family history of smoking-related factors were not evaluated.
The study excluded children who started antiretroviral therapy and developed tuberculosis within a month, potentially underestimating the incidence of tuberculosis.
Since the study was conducted at the tertiary level of healthcare (at comprehensive specialised hospitals), there may be biases related to healthcare accessibility.
Introduction
Tuberculosis (TB) remains an important public health problem, particularly in low-income and middle-income countries (LMICs).1 Globally, the incidence of TB in 30 high-burden countries accounts for 87% of cases.2 Children and young adolescents (aged below 15 years) account for 1.25 million (12%) of the total TB incidence across the globe every year.3
The risk of developing this global infectious disease is 18 times higher among HIV-infected individuals.4 It remains the second leading cause of death from infectious diseases, with the risk of death threefold higher among HIV-infected individuals worldwide.1 4 Recently, the global occurrence of TB mortality in children has increased significantly, which was 214 000 in 2023. Each day, 600 children lose their lives to this preventable disease. Approximately 31 000 deaths are related to people with HIV.2 5
In Sub-Saharan African countries, the HIV-TB burden has increased despite the test-and-treat policy in the region. The incidence rate of TB among HIV-infected children is 3.42 per 100 person-years.6 This problem has a significant economic burden in LMICs.1 Similar to other Sub-Saharan African countries, TB remains a major health issue among HIV-infected children in Ethiopia, with an incidence rate of 4.33 cases per 100 person-years.7
The rate of occurrence of TB among children on antiretroviral therapy (ART) is 24.2%–32% in Nigeria8–10 and 15.2% in Ethiopia,11 while the incidence of TB among children with HIV varies from 0.83 to 2.3 in China,12 7 per 1000 person-years in Thailand,13 4 in South Africa,14 2–2.63 in Ethiopia15–17 and 17.4 in East Africa.18 Some studies have shown that severe immunosuppression, age less than 2 years, WHO clinical staging,19–21 being underweight,21 a low cluster of differentiation 4 (CD4) cell count, anaemia22 and virological failure23 contribute to the occurrence of TB among children on ART treatment. To better understand the variety of contexts and the application of new approaches in factors that affect the risk of TB in children living with HIV, additional investigation is necessary.
The achievement of Ethiopia’s Sustainable Development Goals and the End TB Strategy targets established by the WHO and the United Nations (UN), which aim to reduce TB cases by 80% and deaths from TB by 90%, provide support for this study.3 24 The political declaration of the UN high-level meeting on TB held in WHO plans to reach TB preventive treatment (TPT) at least 90% of people with HIV in 2027.25 Ethiopia has adopted a test-and-treat strategy since 10 June 2014 for all children initiating ART regardless of CD4 cell count and WHO clinical staging, aiming at reducing HIV-related mortality and morbidity.26 Despite this intervention, there is a paucity of evidence on the overall incidence of TB in children in Amhara Region after the test-and-treat strategy.
In addition to previous studies, this finding covers a wide range of public institutions, and rapid initiation of ART and dolutegravir (DTG)-containing ART drugs was incorporated as independent variable to determine the incidence of TB. The current study population also consists of newly enrolled paediatric HIV care patients, rather than patients ever enrolled on ART. Timely and recent evidence is very crucial, and more research is required to incorporate these variables and new strategies. The findings will help decision-makers and policymakers at different levels of HIV/AIDS care and assistance. Therefore, this study aimed to assess the incidence of TB and its predictors among HIV-infected children enrolled in ART care in Amhara Region.
Methods
Study design, setting and period
A multicentre institution-based retrospective follow-up study was conducted among HIV-positive children enrolled in care at Amhara Region’s comprehensive specialised hospitals from July 2014 to March 2022. In Ethiopia, comprehensive specialised hospitals represent the highest level of healthcare facility (tertiary level of health services) and provide advanced medical services, specialised care and training centre for health professionals.
Amhara Region is located in the northwestern, northeastern and north-central parts of Ethiopia, with an estimated area of 159 173.66 km2. The population in this region was most recently estimated at 30 848 988.27 In this region, there are 81 hospitals, of which 8 are comprehensive specialised and teaching hospitals: University of Gondar, Tibebe Ghion, Felege Hiwot, Debre Markos, Debre Tabor, Dessie, Woldia and Debre Birhan. These hospitals have been providing ART care and support as part of the National AIDS Control Program. Of the total number of comprehensive specialised hospitals, seven were included in the study; Tibebe Ghion was excluded due to an insufficient number of study participants. A total of 1095 children were newly enrolled in HIV care in Amhara Region from July 2014 to March 2022.
Population and eligibility
The source population for this study comprised all HIV-infected children who were enrolled in the paediatric HIV care clinics of the comprehensive specialised hospitals in Amhara Region and had a minimum of 1 month of ART follow-up. HIV-positive children who were newly enrolled in the paediatric HIV care clinic in Amhara Region from July 2014 to March 2022 were the study population. Children with HIV who already had TB before starting ART and children with incomplete records of baseline information (CD4 count, WHO staging, haemoglobin level, weight and height) and with unknown dates of ART initiation and TB occurrence were excluded from the study (online supplemental figure 1).
Supplemental material
Sample size determination
The sample size was determined based on key predictors, including height for age, WHO clinical staging and ART adherence level. Using Stata V.17 and the Cox model, the calculation was performed under the following assumptions: a critical value (Z ) of 1.96 for a 5% significance level, a critical value (Z
) of 1.96 for a 5% significance level, a critical value (Z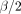 ) of 0.84 for 20% power, an event probability of 0.16715 and a probability of withdrawal of 0.15 using adjusted HR (AHR) (table 1).
) of 0.84 for 20% power, an event probability of 0.16715 and a probability of withdrawal of 0.15 using adjusted HR (AHR) (table 1).
Estimated sample size determination by predictor variables for HIV-infected children on ART using Stata V.17 and Cox model
Sampling procedures and sampling technique
From the calculated sample sizes, the largest sample size (430) was selected as the sample for this study. The sample was allocated proportionally to the comprehensive specialised hospitals in Amhara Region, and records were selected using simple random techniques (online supplemental figure 2).
Supplemental material
Variables of the study
The outcome variable for this study was the occurrence of TB during the follow-up period. The independent variables were sociodemographic characteristics including age, sex, residence, current parent’s status, educational status of the caregiver, HIV disclosure status and marital status of the caregiver. Baseline clinical, nutritional and laboratory characteristics include CD4 count, WHO clinical staging, haemoglobin level, anthropometric indices, previous opportunistic infections, and functional and developmental status. ART and medication-related characteristics include baseline ART regimen, DTG-containing ART drugs, regimen change, level of ART adherence, TPT, cotrimoxazole preventive therapy (CPT), ART side effects and initiation of ART.
Operational definition of the variables
Events: the occurrence of TB among HIV-infected children during the follow-up period at any time after enrolment in the paediatric HIV care clinic, as confirmed by a healthcare professional.
Tuberculosis: defined as a diagnosis of either pulmonary tuberculosis or extrapulmonary tuberculosis as documented on children’s medical records during the study period.
Censored: children who were lost to follow-up, dropped out, transferred out, died due to any cause or completed the study period before developing TB.
Pulmonary tuberculosis: diagnosed based on the national TB diagnosis guideline and primarily affects the lungs, and diagnosis is based on clinical symptoms, chest X-ray findings, sputum smear microscopy or molecular tests such as GeneXpert, or histopathology.
Extrapulmonary tuberculosis: defined as TB that affects organs outside the lungs, such as lymph nodes, pleura, bones and joints, meninges or the abdomen, and diagnosis may involve biopsy, imaging or fluid analysis.
Wasting: if the weight for height Z-score is less than −2 SD for less than 5 years, or if the body mass index for age Z-score is less than −2 SD for greater than 5 years.28
ART adherence levels: categorised as follows: good (≥95% compliance or ≤3 missed doses per month), fair (85%–94% compliance or 4–8 missed doses per month) and poor (<85% compliance or ≥9 missed doses per month), as recorded by ART health personnel.28
Rapid initiation of ART: refers to starting ART on the same day as HIV diagnosis or within 7 days afterwards.29
Urban areas: officially designated towns with municipal structures and better access to services like education, healthcare, electricity and water.30
Rural areas: primarily agricultural or pastoral, with limited infrastructure such as roads, electricity, healthcare and schools, and characterised by dispersed settlements.30
Anaemia: defined as having a haemoglobin level of less than 10 mg/dL.31
Data collection tool and procedures
Data were retrospectively collected using a data extraction tool based on Ethiopian ART guidelines. Information was obtained from ART intake forms, follow-up forms and children’s medical charts for patients newly enrolled at ART clinics from July 2014 to March 2022.28 Data were extracted for 1 month, from 17 May to 15 June 2022. The variables included sociodemographic, clinical, laboratory, ART-related and medication-related factors. Data collection was conducted by seven bachelor’s degree nurses who had smartphones and prior experience with ART follow-up, as well as basic training in ART management.
Data quality control
The data extraction tool was pretested on 5% of the sample size at the University of Gondar Comprehensive Specialized Hospital prior to the actual data collection. Additionally, 1-day on-site training was conducted for data collectors and supervisors, covering topics such as reviewing ART follow-up and medical records, data collection techniques and the study’s objectives. Data collection was carried out using the KoBo Toolbox, which was prepared with relevant restrictions by trained nurses working in hospitals. Additionally, the data collector and principal investigators carefully monitored the entire data collection process and daily submission reports.
Data processing and analysis
The KoBo Toolbox32 was used for data collection, and data were then exported and imported into Stata V.17 statistical software33 for final analysis. Additionally, anthropometric indices were created using the WHO Anthro and WHO AnthroPlus software. To describe the data, descriptive statistics including mean (±SD), percentage and frequency were employed. The variance inflation factor (VIF) was used to check the association between predictor variables; the average VIF was found to be 1.18, which indicates no significant multicollinearity.
A separate graph of Kaplan-Meier survival functions and the log-rank test were estimated for each categorical variable to compare survival between different exposure groups. The proportional hazard assumption (PHA) was checked using both graphical and statistical global tests, which revealed that the PHA was satisfied. Log-likelihood and Akaike information criteria (AIC) were applied to select the best-fitted model, and the model with a minimum AIC was considered the best-fitted model.
In our survival analysis, we considered parametric models including exponential, Weibull, log-normal, log-logistic and Gompertz regression models. Models were evaluated based on the AIC to identify the best fit. The Gompertz model, with the lowest AIC value of 236.02, was selected as the best-fitted model. This indicates that among the models considered, the Gompertz model provided the best balance between model fit and complexity.
In addition, the goodness-of-model fitness was checked using the Cox-Snell residual test. Variables with a p value less than 0.25 in the bivariable analysis were fitted into the multivariable Gompertz regression model. HR with 95% CI was used to determine the strength of the association. Variables with a p value less than 0.05 in the multivariable analysis were considered statistically significant.
To handle and manage missing data, multiple imputations using multivariable chained equations were carried out. The variables missing at random were clarified by the ‘Little test’ results for continuous variables and graphical patterns for categorical variables, making the application of multiple imputations straightforward. Furthermore, sensitivity analysis was performed using both descriptive and inferential statistical techniques to confirm if a significant difference was detected between the outputs of the original and imputed data.
Patient and public involvement
Patients and the public were not directly involved in the design, conduct and dissemination of this study.
Results
Sociodemographic characteristics
A total of 430 medical records of HIV-infected children on ART were reviewed. Of these, 421 (97.9%) were included in this study, with the remaining excluded from the analysis due to data incompleteness. The mean (SD) age of the study participants was 7.58 (±4.02) years; one-third were found to be in the 5–9 years age group. Nearly two-thirds (58.43%) were male and 70% were from urban areas (table 2).
Sociodemographic characteristics of HIV-infected children on ART at comprehensive specialised hospitals in Amhara Region, Ethiopia, 2022 (N=421)
Baseline clinical, laboratory, nutritional and ART medication-related characteristics
At baseline, 79.9% of children were in WHO clinical stage I or II. The median CD4+ count was 421 cells/L (IQR: 403, 1017 cells/L), with one-fifth (20.19%) having CD4+ below the threshold level. The median haemoglobin level was 12.3 (IQR: 10.7, 13.6) mg/dL, with 17.34% having haemoglobin level less than 10 mg/dL. Nearly 30% and 50% of children were wasted and stunted at baseline, respectively. Out of 421 HIV-infected children, 296 (70.31%) had a good ART drug adherence level. More than two-thirds (63.42%) of children had never received TPT and 45.13% had never taken ART within 7 days of its duration (online supplemental table 1).
Supplemental material
Follow-up time and incidence of TB
A total of 421 children were followed from 1 to 89 months. The mean follow-up time was 42 months (14.57–70.07 months). The total time at risk was 1484.75 child-year observations (CYO). New TB cases were observed in 32 (7.6%) children; the overall TB incidence rate was 2.16 (95% CI 1.52, 3.05) per 100 CYO (figure 1).
Overall Kaplan-Meier estimate of tuberculosis (TB)-free probability in children on antiretroviral therapy at comprehensive specialised hospitals in Amhara Region, Ethiopia.
The incidence rate of TB was 3.98 (95% CI 1.99, 7.95) per 100 CYO in the 6 months of ART initiation and decreased to 0.33 (95% CI 0.05, 2.34) per 100 CYO after 3 years of ART initiation. The cumulative probability of TB incidence at the end of 2, 4 and 6 years of the study was 0.9636, 0.9309 and 0.9045, respectively. The incidence rate of TB was 8.09 (95% CI 5.03, 13.01) per 100 CYO among children with anaemia, while it was 1.18 (95% CI 0.7, 1.95) per 100 CYO among those without anaemia (online supplemental figures 3–6).
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Model comparison
The PHA, semiparametric and parametric proportional hazard models were fitted to estimate the incidence of TB and its predictors among HIV-infected children in Amhara Region. The graphical test and global test (p=0.2980) confirmed the PHA. The most fitted model was chosen using AIC (236.02), Bayesian information criteria (304.74) and log-likelihood (−138.43). The results outperformed the Cox proportional hazard and other parametric models in all three comparison techniques. As a result, interpretations and conclusions were based on the Gompertz model (online supplemental table 2).
Supplemental material
The Cox-Snell residual test checked the goodness of fit (figure 2), shared frailty was estimated (theta: 1.89e−07), and the distribution of unmeasured variables is indifferent between the comprehensive specialised hospitals.
The goodness-of-fit test for the Gompertz regression model of the incidence of tuberculosis among children on antiretroviral therapy at comprehensive specialised hospitals in Amhara Region, Ethiopia.
Predictors of incidence of TB
In the bivariable parametric survival Gompertz regression, parental status of the caregivers, educational status of the caregivers, baseline WHO clinical staging, CD4+ count, haemoglobin status, functional and developmental status, regimen change, ever taking TPT, ever taking CPT, ART adherence level, previous opportunistic infections, DTG-containing ART drugs, wasting and rapid initiation of ART were found to be significant predictors of TB among children on ART. In the final multivariable Gompertz regression model, only baseline haemoglobin, ever taking TPT, wasting and rapid initiation of ART significantly increased the incidence of TB.
Children with anaemia had 3.83 times (AHR: 3.83; 95% CI 1.46, 10.04) higher risk of acquiring TB infections than children without anaemia. Likewise, the risk of developing TB in children who were not given TPT was 3.78 times (AHR: 3.78; 95% CI 1.44, 9.94) higher compared with those who were given TPT at baseline. Additionally, the hazard of TB among children with wasting at baseline was 2.53 times (AHR: 2.53; 95% CI 1.19, 5.38) more likely compared with their well-nourished counterparts. Furthermore, children who had late ART initiation had 3.27 times (AHR: 3.27; 95% CI 1.26, 8.46) increased risk of developing TB compared with those rapidly initiated on ART (table 3).
Bivariable and multivariable Gompertz Cox regression analyses of predictors of tuberculosis among children on ART at comprehensive specialised hospitals in Amhara Region, Ethiopia, 2022 (N=421)
Discussion
This study aimed to assess the TB incidence rate and its predictors among children on ART in Amhara Region’s comprehensive specialised hospitals.
In this study, the incidence of HIV-infected children on ART was 2.16 (95% CI 1.52, 3.05) per 100 CYO, which is consistent with the studies conducted in Debre Markos at 2.63 per 100 CYO,16 in Northeast Ethiopia at 2.0 per 100 CYO34 and in Southern Ethiopia at 2.6 per 100 CYO.17 This finding is lower than the studies conducted in Northern Ethiopia,35 Benishangul Gumuz region in Northwest Ethiopia,11 36 Adama Referral Hospital and Medical College,37 Southwest Ethiopia38 and Tanzania.39 This could be due to the higher burden of TB in resource-limited settings,40 and might also be due to differences in sociodemographic and baseline clinical characteristics of the study participants, as well as changes in study period and setting. However, the incidence in our study is higher than the studies conducted in New York City,41 Ireland and the UK42 and China.12 This could be because the general population in high-income countries has a low incidence rate of TB. In addition, the use of early TB diagnosis and prevention techniques, along with accessibility of modern technologies, significantly lowers the incidence of TB.43 Furthermore, there are higher cases of TB in Ethiopia due to poverty, overcrowding, large families and subpar living conditions.
Children who were classified as having anaemia had 3.83 times higher risk of acquiring TB infections than children without anaemia. Supported by studies in Northern Ethiopia35 and Benishangul Gumuz,36 this high prevalence of TB cases in anaemia is due to persistent inflammation or chronic diseases.44 Anaemia also appears to have predictive value for incident TB disease during ART.45
Likewise, the risk of developing TB in children who were not getting TPT was 3.78 times higher compared with those who were taking TPT at baseline. This finding is supported by different studies conducted in Ethiopia,35 46 Tanzania47 and by global data.48 TPT decreases Mycobacterium load and reduces the progression of latent TB bacilli to active TB. Increases in mycobacterial load are associated with progressive impairment of Mycobacterium specific T-cell responses and increase the occurrence of active TB.49 Additionally, the synergetic effect of ART and TPT in reducing the incidence of active TB increases as a result of CD4+ T-cell recovery and viral load suppression.25 Lower TPT intake is associated with an increased incidence of TB; in our study, only 63.42% of children with HIV took TPT.
The hazard of TB among children with wasting at baseline was 2.53 times more likely compared with their counterparts. This finding is supported by studies conducted in Northern Ethiopia, Debre Markos16 35 and Southern Ethiopia.50 This could be explained by the fact that malnutrition causes nutritionally acquired immune dysfunction and increases the host’s susceptibility to infections. Undernutrition also weakens the immune system through atrophy of the thymus, spleen and lymph nodes and reduces cell-mediated immunity.51 52 Immunological and clinical recoveries are directly associated with nutritional status.53
Furthermore, children with late ART initiation had 3.27 times increased risk of developing TB when compared with those who had rapid ART initiation. This finding is in agreement with a study done in Ethiopia.16 Initiation of ART early after confirmation of HIV infection, even at higher CD4 cell count, helps reduce the occurrence of TB. This could be attributed to the early initiation of ART, which helps minimise delays, enhances viral suppression rates in children with HIV, improves retention in care and reduces the risk of HIV transmission.54
This study was conducted at tertiary-level comprehensive specialised hospitals, which may introduce bias related to healthcare accessibility, particularly in LMICs with significant geographical and socioeconomic disparities. These hospitals primarily serve urban populations and provide advanced medical services, potentially limiting the generalisability of findings to children in rural areas. A substantial proportion of ART children in the region, especially those with limited access to healthcare, were not represented.
Strength and limitations of the study
As a strength, this study was multicentre, covering wide ranges in geographical areas of specialised hospitals, and used a longer follow-up period to better estimate the incidence of TB. There are certain inherent limitations to the current study. Owing to financial limitations, factors such as housing characteristics, the environment and family history of smoking-related factors were evaluated retrospectively rather than prospectively in order to determine the incidence and predictors of TB following the start of ART. Additionally, the study excluded children who began ART at the outset and acquired TB within a month or less. This may have led to an underestimation of the incidence of TB.
Conclusion and recommendations
In this study, the incidence of TB was found to be high. Anaemia, wasting, TB preventive therapy and early initiation of ART were factors found to be significant predictors of TB incidence in HIV-infected children receiving ART. Additionally, clinicians should emphasise early screening and maximise nutritional supplements for HIV-infected children to decrease the incidence of TB in these individuals. Furthermore, emphasis is needed on early detection and treatment of anaemia as well as on TB preventive therapy, counselling on nutritional improvement and timely initiation of ART to avert TB. Future studies should include data from community-level health facilities to ensure more representative and equitable findings.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study used secondary data or medical charts; the study was approved by the Institutional Review Board of the University of Gondar College of Medicine and Health Sciences, on behalf of the Ethical Review Committee of the School of Nursing (SN/212/2022), with a waiver of informed consent. Before collecting data, a letter of permission was obtained from each comprehensive specialised hospital administrator to collect the data from each ART clinic. At the time of abstraction, personal identifiers (names and contact numbers) were excluded. The data were kept strictly confidential and used only for study purposes. All procedures were carried out considering the Declaration of Helsinki.
Acknowledgments
The authors acknowledge the University of Gondar for ethical clearance, as well as each comprehensive specialised hospital and the data collectors.
References
Footnotes
Contributors GBM worked on developing the research idea and designing the study, and was involved in proposal writing, training and supervising the data collectors, analysing and interpreting the results, and preparing the manuscript. BTL, FDB and WTW participated in critically revising the proposal, designing the study, analysing and interpreting the results, and writing the manuscript. All authors were involved in reading and approving the final manuscript. GBM is responsible for the overall content as the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.




