Article Text
Abstract
Introduction Acute pain levels following orthopaedic injury (eg, fracture) are a predictor of the onset of chronic pain, which affects nearly 50% of fracture patients and impairs functional recovery. Among current pharmacological treatments for acute pain, non-steroidal anti-inflammatory drugs have been associated with delayed bone healing, while opioids inhibit effective bone remodelling, increase the risk of pseudarthrosis and carry a high risk of addiction. In light of this, the development of new pain treatments is essential. Cannabidiol (CBD), a non-addictive and non-psychotropic cannabis component stands out as a potential therapeutic agent, given its analgesic and anti-inflammatory properties as well as its potential benefits for bone healing. This randomised controlled trial aims to investigate the effect of acute CBD treatment, compared with placebo, on patients’ self-reported pain, inflammation and well-being following a fracture injury.
Methods and analysis This is a triple-blind, randomised, placebo-controlled clinical trial. A total of 225 adults aged 18–70 years, who have suffered a long bone fracture and were treated at the Hôpital du Sacré-Coeur de Montréal, will be randomly assigned within 1 week to one of three treatment arms (25 mg or 50 mg of CBD or placebo) for 1 month. The primary outcome will be the difference in the pain score between groups at 1-month follow-up. Secondary outcomes will include measures of persistent pain, inflammation, opioid usage, quality of life, sleep quality, depression, anxiety, cognition and orthopaedic function. Data will be collected at baseline, 1-month and 3-month follow-ups.
Ethics and dissemination This study obtained a Health Canada licence for use of cannabis products. It has also been approved by Health Canada and the Research Ethics Board of the CIUSSS du Nord-de-l’Île-de-Montréal (Project ID 2025-2105). The findings will be published in a peer-reviewed journal and presented at local, national and international conferences. The trial’s results will be made publicly available on the ClinicalTrials.gov database.
Trial registration number NCT06448923.
- Clinical Trial
- PAIN MANAGEMENT
- Orthopedics
- Fractures, Bone
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study is robust due to its triple-blind randomised, placebo-controlled design, which assesses the effects of two different doses of pharmaceutical-grade cannabidiol (CBD).
The extensive number of measurements allows for a comprehensive assessment of the treatment’s impact, not only by evaluating patients’ perceived well-being and recovery but also by objectively quantifying CBD’s effect on inflammation through inflammatory markers.
This trial includes a longitudinal assessment of CBD treatment on pain symptoms and trauma-related outcomes up to 3 months postfracture, a critical period marking the transition to chronic pain, however, the long-term effects of the treatment will not be assessed.
A limitation is the exclusion of osteoporotic patients, as well as a potential restriction in the inclusion of women since those of childbearing age who are not using contraception will have to be excluded due to limited knowledge on the teratogenic effects of CBD.
Another limitation of this study is that therapeutic drug monitoring was not performed, which could have helped account for interindividual variability and optimise dosing.
Introduction
Bone fractures are a prevalent condition affecting individuals of all ages and are the most commonly treated trauma in hospitals.1 2 In 2019, the estimated annual incidence of new fractures worldwide was 178 million.3 The process of bone healing involves multiple consecutive and interrelated phases including inflammation, repair and remodelling, which occur in a spatial and temporal series of dynamic processes.4 5 The skeletal system possesses a remarkable capacity for regeneration. The initial process of bone healing typically occurs over a period of 8 weeks,6 while bone remodelling extends for months following a fracture.7
Independent of body location, traumatic injury sets off an acute non-specific immune response characterised by the release of proinflammatory cytokines such as interleukins (IL-1β, IL-6, IL-10) and the tumour necrosis factor (TNF-α).8 In addition, systemic acute inflammation after bone fracture promotes the sustained release of cytokines disrupting the blood–brain barrier, thereby allowing toxic intruders such as proinflammatory cytokines to invade/migrate to the central nervous system (CNS).9 Persistent CNS inflammation plays a key mediating role in central sensitisation,10 a maladaptive plasticity process driven by an increased response to nociceptive inputs, involved in pain persistence and chronicity. Chronic pain, a condition associated with delayed functional recovery, sleep disturbances, mental health disorders and poorer quality of life,10 is highly prevalent 3–6 months after trauma, affecting 30%–50% of individuals with bone fractures.11 A number of variables have been identified as potential predictors of chronic pain after trauma, including pain intensity at 3 months postaccident, female sex, poor sleep, levels of anxiety and depression, and the concomitant occurrence of traumatic brain injury (TBI) or peripheral nerve injury at the time of fracture.12–16
Following a fracture, patients frequently report a range of symptoms, including increased fatigue and motor impairment, which can exert a significant impact on their ability to perform activities of daily living.17 In addition, patients with orthopaedic trauma report a deterioration in their quality of life up to 12 months following the injury.17 18 However, pain emerges as the most prominent complaint, with 97% of patients reporting pain after an orthopaedic injury.1 19 Acute pain management is a crucial concern considering that inadequate pain control can lead to prolonged inflammation, which can perpetuate pain signals and lead to chronic pain.20 21 Currently, a pharmacological approach is widely recommended to manage acute post-trauma pain. Both non-steroidal anti-inflammatory drugs (NSAIDs) and opioids are frequently prescribed for their anti-inflammatory and analgesic effects.22 Nevertheless, the use of NSAIDs has been associated with delayed bone healing23 24 as well as digestive complications and kidney failure.25 As for opioids, in addition to major side effects, they pose a high risk of dependence and tolerance.4 26 Furthermore, several studies show that opioids inhibit effective bone remodelling,27 increase the risk of pseudarthrosis28 and heighten the risk of hyperalgesia, that is, a paradoxical increase in pain sensitivity due to central sensitisation.26
Interestingly, following the legalisation of cannabis in Colorado, a reduction in chronic pain admissions was observed, leading experts to question the potentially beneficial effects of cannabis on pain.29 Indeed, one study found that 61% of medical cannabis users reported consuming it to alleviate pain.30 31 However, the medical use of cannabis is limited due to the undesirable psychotropic and addictive effects of tetrahydrocannabinol (THC). Cannabidiol (CBD), an organic component of cannabis, is non-psychoactive due to its low affinity with the CB1 receptor.32 It is of particular interest as it is devoid of addictive effects33 34 and has an excellent safety profile,35 and its use does not affect daily activities such as driving or working.
CBD is highly lipophilic which facilitates its ability to cross the blood–brain barrier.36 However, the bioavailability of CBD varies greatly according to the method of administration. The bioavailability of oral CBD is lower due to the hepatic first-pass effect, with approximately 5% of the product reaching the bloodstream.37–39 Food consumption as well as nanotech and oil-based formulations of CBD have been shown to increase bioavailability.40 However, compared with smoked CBD, oral administration of CBD presents multiple advantages, including greater control over dosage, ease of administration and fewer side effects.38
Mechanisms of action of CBD are complex, not yet fully understood and involve multiple pharmacological targets. Emerging evidence suggests that CBD exerts a number of important effects via its modulating role on several non-cannabinoid receptors and ion channels including those of endogenous neurotransmitters, such as serotonin41 as well as several types of transient receptor potential channels (TRP), such as TRPV1,42 and by modulating the binding affinity of certain G protein-coupled receptors.43 Several in vitro and animal model studies have demonstrated CBD’s anti-inflammatory effect, notably by reducing proinflammatory cytokines such as TNF-α, IL-1β and IL-6, in addition to inhibiting microglial activation.32 42 44–51 CBD has also shown analgesic potential in studies using neuropathic and inflammatory pain models. These human and animal studies suggest a reduction in pain, hyperalgesia and allodynia following treatment with CBD.35 52–59 CBD is alleged to possess anxiolytic and antidepressant properties, as shown in several animal and human studies.60–66 In addition, a well-controlled preliminary animal study showed that CBD, but not THC, enhanced the biomechanical properties of healing mid-femoral fractures in rats, supporting a beneficial effect of CBD on bone healing.67
Epidemiological studies have suggested a reduction in opioid use for pain coinciding with an increased use of medical cannabis,30 a trend also documented in Canada.68 While the interaction between CBD and opioids is not yet fully understood, studies have shown that CBD acts as an allosteric modulator of the mu-opioid and delta-opioid receptors.69 CBD was also shown to potentially enhance the analgesic effects of endogenous and exogenous opioids. In one study, the use of CBD as a coanalgesic treatment for patients with chronic pain resulted in a reduction in opioid consumption and improvements in overall quality of life.70
Providing effective pain management for patients with fractures is not only a fundamental right but also offers numerous benefits. It reduces stress, shortens hospitalisation time, decreases associated healthcare costs and lowers the risk of developing chronic pain.1 Preventing chronic pain is easier than reversing the sensitisation processes that cause it,71 making acute pain control a priority. Given its excellent safety profile33 35 coupled with its downregulating effects on microglial and inflammatory activity, the primary neuroinflammatory and pain mechanism, CBD represents an appealing neuroprotective agent for pain-susceptible orthopaedic trauma patients.
Study objectives
The primary objective of this study is to evaluate the effects of CBD treatment on self-reported pain in patients following a long bone fracture injury. The second objective is to assess the effect of the CBD treatment on inflammation and patient well-being. Additionally, secondary analyses will look at the possible associations between pain mediators (such as opioids, sex and mild TBI (mTBI)) and response to CBD treatment. The aim is to better identify the effects of these pain mediators on treatment response and the impact of CBD treatment on opioid uptake.
Methods
Study design
This is a randomised, placebo-controlled, triple-blind 1-month clinical trial evaluating the effects of two doses (low and moderate) of CBD compared with a placebo on pain and inflammation after a long bone fracture.
Participants
A total of 225 participants aged 18–70 will be recruited within 1 week after their long bone fracture injury and consultation to the Hôpital du Sacré-Coeur de Montréal (HSCM), one of the largest level 1 trauma centres in Canada with approximately 3500 orthopaedic patients treated annually. The planned age range, targeting a population representative of individuals who frequently experience traumatic fractures, was chosen as it allows for a more homogenous evaluation of fracture healing and pain recovery. Including participants over 70 introduces additional challenges, such as increased comorbidities, chronic diseases, bone fragility, polymedication and increased complications, which could slow the healing process, influence pain perception and complicate result interpretation.
Inclusion criteria
Subjects meeting the following criteria are eligible for the trial:
Patients with a long bone fracture of the lower limb (tibia, fibula, femur, metatarsals and phalanges) or the upper limb (humerus, radius, ulna, metacarpals and phalanges) treated to HSCM within 1 week of the accident.
Participants are between 18 and 70 years of age.
Patients with or without surgical procedures.
Exclusion criteria
Patients presenting any of the following characteristics are not eligible for the trial:
Moderate/severe TBI.
Diagnosis of any of the following mental disorders as defined by the Diagnostic and Statistical Manual of Mental Illnesses (DSM-5): schizophrenia, intellectual disability, bipolar disorder, major depression, a diagnosed and untreated sleep disorder.
History of alcohol or opioid misuse/abuse, as defined by the DSM-5.
Evidence of severe renal (stage 4 or 5) or hepatic impairment (Child B or C).
Pregnant or lactating women, women of childbearing potential who are not using medically accepted forms of contraception (eg, condoms, oral contraceptive or intrauterine device) or women who are actively planning on becoming pregnant.
History of adverse reactions to cannabis.
Patients taking warfarin, sildenafil, valproate or under opioid treatment prior to the injury.
Patients experiencing, on average, mild-to-absent pain in the last 24 hours preceding recruitment (as per a score<30 on a 0–100 mm Visual Analogue Scale (VAS)).
Transport business drivers and heavy machinery operators.
A diagnosis of chronic pain, bone pathology (eg, osteoporosis) or chronic inflammatory disease (eg, rheumatoid arthritis, arthritis, psoriasis).
Not having French or English as a spoken language.
A weighted Montreal Cognitive Assessment (MoCA) score of less than 24.
Regular cannabis use more than five times a week.
Recruitment
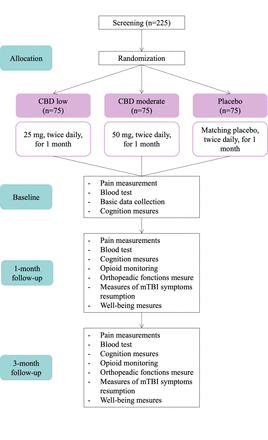
Recruitment will begin in January 2025 and end in January 2027. Potential participants will be screened daily by the research team and collaborators. Potentially eligible patients will be approached by a research team member and provided with a consent form. Once the research team has addressed any remaining questions and obtained a signed consent form, the participant will be randomised. See figure 1 for an overview of the study timeline.
Study schema. CBD, cannabidiol; mTBI, mild traumatic brain injury.
Assignment of interventions
Participants will undergo concealed randomisation to avoid selection bias. The study pharmacist will randomly assign participants to one of the three treatment groups (low or moderate CBD or placebo) using a 1:1:1 ratio through block randomisation with randomly selected block sizes (9 and 12), stratified by sex, age (ie, participants aged 45 and under, and those over 45) and type of fracture (ie, lower and upper limb). Block randomisation with randomly selected block sizes (9 and 12) was chosen to minimise selection bias and maintain the blinding of investigators and other project members by ensuring the unpredictability of block assignments. Given that there are three groups, a block size of 9 results in a distribution of 3 patients per group, whereas a block size of 12 allocates 4 patients in each group. The randomisation procedure will be performed a priori by an independent biostatistician. Identical tablets for CBD and placebo will ensure blinding of clinicians, researchers, patients, families, and biostatisticians to avoid unequal co-interventions, ascertainment bias, and analytic bias. The study pharmacist will be aware of allocation but will have no clinical or interpretive role. Assignments will be kept in sequentially numbered, sealed envelopes to ensure adequate allocation concealment. In the event of a serious adverse event or reaction, the allocation list can be retrieved.
Intervention
Patients in the treatment group will receive either a low dose (25 mg per tablet) or a moderate dose (50 mg per tablet) of CBD self-administered orally as a tablet twice daily with a meal for 1 month. Patients in the placebo group will receive an identical amount of a matching placebo administered with a meal twice daily for 1 month.
Investigational product
CBD tablets, along with matching placebos, will be supplied by EmpowerPharm (Toronto, Canada). The pharmacokinetic profile of the CBD product has already been established, and efforts to register the product with Health Canada have begun.
Dose justification
A wide range of CBD doses ranging from<1 to 50 mg/kg has been used in various conditions.72 73 The low dose (25 mg) selected for our study is based on initial and ongoing studies of CBD in chronic pain which used a mean dose of 22.5 mg and 20 mg per day.65 This is also approximately the mean dose of CBD administered in a successful trial of Sativex (THC/CBD) for neuropathic pain.74 Moreover, a higher but relatively moderate dose of CBD (50 mg) will be evaluated to assess dose-response effect. CBD doses in this range have shown no statistical difference in intoxication scores in healthy volunteers,75 and doses of up to 800 mg per day for a minimum of 4 weeks showed an excellent safety and tolerability profile.76 77 Participants will be advised to ingest the treatment at mealtime, as studies have shown an increased bioavailability of CBD in subjects after eating.78 79 To achieve our primary goal of mitigating acute pain, a 1 month treatment period has been selected, aligning with the typical evolution of acute pain post-fracture.6
Study procedure
On enrolment in the study, research staff will provide study instructions, collect baseline data (eg, demographics and clinical characteristics), administer questionnaires and cognitive tests, and collect blood samples for quantification of pro-inflammatory cytokines. Patients will report their pain intensity at baseline and then three times a week throughout the entire 1 month treatment duration. Participants will be instructed to complete a daily medication diary for 1 month to monitor the administration of study product, as well as opioid drugs or other analgesic medication use. This data will be collected via questionnaires sent by email or SMS message from the REDCap secure database. At 24 hours following treatment completion (1-month follow-up) and at the 3-month follow-up, participants will be evaluated at the research laboratory to collect measures of pain intensity and related outcomes including opioids intake, inflammation, cognition, orthopaedic function and indicators of overall well-being. Participants will have to abstain from CBD consumption from the end of treatment until the last follow-up visit. See table 1 for a detailed schedule of assessments.
Schedule of assessment
Primary outcome
The main outcome is the difference between groups in the mean pain intensity score at 1-month follow-up, as measured by the VAS.80 Pain intensity on the VAS will be gathered 24 hours following treatment completion. The VAS is a 100 mm line with anchor words ranging from ‘no pain’ to ‘worst imaginable pain’. Participants will indicate the intensity of their pain at that moment by placing a mark along the line.
Secondary outcomes
At 1-month and 3-month follow-ups, persistent pain, opioid consumption, inflammation markers, quality of life, sleep quality, depression, anxiety, cognition, mTBI symptom resolution and orthopaedic function outcomes will be collected. In addition, at baseline, participants will be asked to indicate their level of treatment expectation using the Treatment Expectation Questionnaire,81 a 15-question questionnaire, considering the potential modulation of therapeutic effects by patients’ expectations of treatment.82 83 After treatment completion, participants will also be asked to indicate whether they felt they had received active treatment or placebo.
Measures
Demographic and clinical characteristics
The following information will be collected at baseline to characterise participants: age, sex, height, weight, percentage of adipose tissue using an impedance metre scale, occupation, education level, ethnicity, language spoken, premorbid medical history (including psychological health history), premorbid substance use (eg, alcohol, drugs, cigarettes, medications), recreational cannabis use, history of brain trauma, injury type and severity and mechanism of injury.
Pain
At 1-month and 3-month follow-up, persistent pain will be assessed using the Brief Pain Inventory Short Form,84 a nine-item self-report questionnaire assessing for the presence, intensity and location(s) of pain, as well as perceived efficacy of pain relief treatment, and pain interference with activities of daily living. In addition, pain will be assessed using the VAS at several time points for comparison: baseline, three times per week during treatment, 24 hours after the end of treatment and at the 3-month follow-up. Pain catastrophising will also be assessed during the initial visit using the Pain Catastrophising Scale,85 a 13-item questionnaire evaluated on Likert scales, given the significant contribution of psychological factors in the experience of pain.
Opioid usage
Participants will continue their usual pain care regimen throughout the study. Opioid usage and analgesics will be recorded in a daily medication diary for the initial month and through the number of prescription refills for months two and three. Self-reported opioid use in a diary has been shown to be an accurate assessment of the quantity of opioids consumed.86
Inflammation
Blood levels of proinflammatory cytokines including interleukins (IL-6, IL-10, IL-1β) and TNF-α will be collected at baseline and at the 1-month and 3-month follow-up sessions. To assess cytokine levels, blood samples will be separated in buffy coat, serum and plasma and stored at −80°C in polypropylene tubes on average 1–2 hours after the blood draw. EDTA plasma will be tested with cutting-edge ultra-sensitive Quanterix ImmunoAssay Analyzer Simoa HD-X to quantify biomarkers using the Cor-Plex-Cytokine-10-Plex assay panel as per manufacturer recommendation. Simoa is a leader in the quantification of plasma biomarkers with markedly lower detection threshold than traditional ELISA.87
Cognition
At baseline, 1 and 3 months follow-ups, neuropsychological tests highly sensitive to pain, and that do not require the use of the fractured limb, will be administered: a task assessing information processing speed (Symbol Search from the Wechsler Adult Intelligence Scale (WAIS-IV) Battery), two memory tests (California Verbal Learning Test and Digit Span from the WAIS-IV battery), two executive function tests (D-KEFS Colour-Word and Verbal Fluency) and an attention test (Elevator counting with distraction and Elevator counting without distraction from the Test of Everyday Attention battery) (see Lezak et al,88 1995 for test descriptions).
mTBI symptoms resolution
Patients who sustained a concomitant mTBI with their fracture will be included in the study. Additional measures will be documented to control for this variable. At 1 and 3 months follow-up, information on mTBI symptoms resolution will be collected for patients diagnosed with mTBI concomitant to the fracture using the Rivermead Post-Concussion Questionnaire.89
Orthopaedic function
At 1 and 3 months follow-ups, the Short Musculoskeletal Function Assessment (SMFA) Questionnaire90 will be administered. The SMFA includes 34 questions that evaluate the patient’s function and 12 questions related to how bothered patients are by their symptoms.
Well-being
At 1 and 3 months follow-ups, various important domains of well-being significantly modulated by pain will be measured including: quality of life using the Short Form (36) Health Survey,91 a 36-item self-report questionnaire for measuring quality of life across 9 domains; sleep quality and quantity using the Pittsburgh Sleep Quality Index,92 a self-report questionnaire that assesses sleep quality and quantity over the past 4 weeks. Additionally, at baseline, 1 and 3 months follow-ups, depression and anxiety symptoms will be assessed using the Beck’s Depression Inventory-II (BDI-II)93 and the Beck’s Anxiety Inventory (BAI).86 The BDI-II is a 21-item multiple-choice self-report questionnaire for measuring depression symptoms. The BAI is a 21-question multiple-choice self-report inventory used for measuring the severity of anxiety. Finally, symptoms of post-traumatic stress disorder (PTSD) will be assessed at the first visit and at 1-month follow-up using the PTSD Checklist for DSM-5 questionnaire.94
Data management
Data collected will be transcribed from the source documents into the electronic case report form (eCRF) on the REDCap database hosted at CIUSSS du Nord-de-l’Île-de-Montréal95 and quality controlled by a second qualified staff member. Data will be stored on a secure network with regular backups. An external, independent clinical monitor will conduct regular monitoring visits according to the monitoring plan, during which they will review and verify source data, informed consent forms, medical records, laboratory results, CRFs, medication dispensing logs and protocol deviations.
Statistical analyses
Sample size estimation
A 30% relative pain intensity reduction on the VAS (expected response of 50% or more in the CBD group and expected 20% in the placebo group) has been used extensively to reflect clinically significant pain relief in clinical trials. Based on a Fisher’s exact test, a sample size of 225 participants (3 groups of 75) will be required to reach a power of 80% to detect a statistically significant difference in the proportion of patients who reach 30% pain reduction between the CBD groups and placebo at 1-month postinjury, assuming a dropout rate of 20% and a significance level of 5%. These parameters are taken from a successful randomised, placebo-controlled clinical trial using Sativex in treating 125 neuropathic pain patients.74
Moreover, considering that the placebo group may ingest more opiates and that the anticipated intergroup effect at 1 month may be reduced to 20%, a total sample size of 225 subjects could be required to achieve 80% power, assuming a drop-out rate of 20% and a significance level of 5%.
Primary outcome
The primary outcome will be analysed using an analysis of covariance, with mTBI and orthopaedic surgery as covariables and treatment (low and moderate CBD vs placebo) as factor in the mean VAS pain score at the 1-month follow-up.
Secondary outcomes
For the secondary outcomes, a Kaplan-Meier survival analysis with the log-rank test on VAS pain data collected during treatment will be used to assess CBD treatment success rate relative to placebo at achieving 50% pain intensity reduction during treatment duration. The proportion of patients no longer experiencing significant pain symptoms at the 3-month follow-up (ie, patients who did not convert to chronic pain) will be compared, as defined as VAS pain ≤30 between treatment with a χ2 test. A mixed model for repeated measures with covariables mTBI and orthopaedic surgery and treatment as a factor will be used to assess between-group treatment effects on total opioid use at both 1 and 3-month follow-ups. The same approach will be used to assess between-group treatment effects at both 1 and 3-month follow-ups on secondary outcome measures listed above.
Analyses will be performed on an intention-to-treat (ITT) dataset. The ITT dataset will include all participants randomised in the analysis, whether or not they have completed treatment in order to limit bias and reflect results under real treatment conditions.
Missing data will be reported and justified in the results. The multiple imputation method, which has been recognised in clinical studies involving experimental treatment, will be applied. Additionally, a sensitivity analysis will be performed to assess the impact of missing data on the results.
Discontinuation
Participants may withdraw from this research project at any time without giving reasons. Discontinuation of treatment does not imply withdrawal from the trial. The following reasons will be considered as grounds for patient withdrawal from the trial: withdrawal of consent by the participant, failure to pass the selection phase, meeting an exclusion criterion, failure to participate in follow-up, termination of the trial by the investigator, major protocol deviation incompatible with trial participation, an adverse event or any other condition which, in the opinion of the investigator, would expose the participant to undue risk by continuing the treatment trial, any condition that the investigator considers medically necessary to withdraw the patient from the trial.
Adherence
During the baseline visit, a research team member will conduct an information session to discuss the significance of adhering to the guidelines related to doses, timing of drug administration, the procedure to be followed in case of a missed dose and the importance of reporting any adverse event. Automatic SMS reminders will be sent to ensure completion of the digital VAS and medication diary. A high protocol adherence is expected given that CBD has limited adverse side effects, and the administration is oral and non-invasive. A 10% loss to follow-up is expected based on a 3-month trial with the same patient characteristics.96 For adherence purposes, patients will be instructed to return all treatment bottles, empty or not, to be monitored by the pharmacy staff. Each participant will receive financial compensation for costs incurred during their participation in this research study. Participants who withdraw or are withdrawn from the project prior to its completion will receive an amount proportional to the length of their participation.
Safety and serious adverse events
Risks of adverse effects are considered low given the demonstrated excellent safety profile of CBD.33 35 Somnolence, fatigue, drowsiness, gastrointestinal issues and decreased appetite are the most probable adverse events associated with CBD in adult patients.34 Participants will be instructed to advise the research team of any adverse events which will be thoroughly monitored and documented. Access to on-duty emergency physicians at HSCM will be provided during the entire treatment duration.
Patient and public involvement
Neither patients nor the public were involved in the development, design and conduct of this study.
Confidentiality
All data collected in our databases will be stored following a deidentification process. Participants will be identified by a unique identification code, and nominal data will be protected separately. Uncoded data will only be accessible to the principal investigator. No identifying data will be disclosed in any scientific communication or publication.
Ethics and dissemination
Ethical approval has been granted by the CIUSSS du Nord-de-l’Île-de-Montréal ethics board (#2025-2105 issued on August 2024) and Health Canada (Licence, #LIC-NKA1EX2TUA-202-3 issued on 26 March 2024 and No Objection Letter, HC6-024-c275232 issued on 30 May 2024). This study adheres to the Declaration of Helsinki. The results will be published in a peer-reviewed journal and presented at local, national and international conferences.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors DB, DW, FB, CA, AMP, DR and LDB conceived the study. DB and AAD will ensure coordination, recruitment and conduct of the protocol. DB and LDB wrote the manuscript. All authors contributed to the revisions of the manuscript. LDB is guarantor.
Funding This work was supported by CIHR (grant #431482), the Caroline Durand Foundation Chair in Acute Trauma and the Complementary Medicine Research & Addiction Foundation. Doctoral training scholarship to DB is provided by the Fonds de recherche du Québec-Santé (BF2–341229). CBD and placebo will be manufactured by EmpowerPharm (Ontario, Canada). The study's design, management, analysis and reporting are entirely independent of the CBD manufacturers.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.