Article Text
Abstract
Introduction The aim of this study is to investigate the effects of acute exercise on appetite control and whether this differs between morning and late afternoon in individuals with overweight/obesity with or without type 2 diabetes (T2D).
Methods and analysis The hedonic and homeostatic appetite control in obesity and type 2 diabetes in the context of meal and exercise timing (TIMEX) study is a randomised, controlled, cross-over trial. Fifty-eight women and men (aged 18–75 years) with overweight or obesity (body mass index ≥25 kg/m2) with or without T2D will be recruited. All participants will complete a screening and baseline visit followed by four test visits: two morning visits and two late afternoon visits. The participants will arrive in the fasted state during the visits. During one morning visit and one late afternoon visit, the participants will engage in a 45-min bout of acute high-intensity interval exercise on an ergometer bicycle. The remaining two visits (one morning and one late afternoon visit) will include 45 min of rest. Fifteen minutes after the rest or exercise period, the participants will be presented with an ad libitum meal. Blood samples will be collected and subjective appetite will be assessed using Visual Analogue Scales in the fasted state before exercise/rest, immediately post-exercise/rest and at 15, 30, 45 and 60 min post-exercise/rest. Food reward and food preferences will be assessed using the validated diurnal version of the Steno Biometric Food Preference Task in the fasted state before exercise/rest and 15 min after the ad libitum meal (45 min post-exercise/rest). The primary outcome is the difference in ad libitum energy intake after exercise compared with rest. Secondary outcomes include eating rate; 24-hour energy intake; appetite-related metabolites and hormones, and circulating biomarkers assessed from proteomics, metabolomics and lipidomics analyses; food choice, food attention and reaction time, explicit and implicit liking and wanting for different food categories, subjective appetite; ratings of perceived exertion during exercise. All outcomes will be compared between morning and late afternoon and between participants with and without T2D.
Ethics and dissemination The study has been approved by the Ethics Committee of the Capital Region of Denmark (H-22019913) and the Capital Region of Denmark’s Research Register (Privacy). The study will be conducted in accordance with the Declaration of Helsinki. All results will be published in national and international peer-reviewed journals and will be disseminated at national and international conferences.
Trial registration number NCT05768958.
- Overweight
- Diabetes Mellitus, Type 2
- Obesity
- NUTRITION & DIETETICS
- Exercise
- Randomised Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Comprehensive assessment of appetite control including various, multidisciplinary methods: ad libitum food intake, subjective appetite ratings, appetite-regulating hormones, assessment of food preferences and 24-hour diet records.
Two-by-two factorial design allows a comparison of the effects of exercise versus rest and the effects of morning versus late afternoon exercise in the same trial.
Cross-over study with a large sample size in people with overweight or obesity with and without type 2 diabetes (T2D).
Heterogenous study population (participants with and without T2D) which increases the generalisability of the results, but also induces larger variation in the data.
No healthy control group (referred to as individuals with body mass index between 18.5 and 24.9 kg/m2) which prevents the study from investigating if the outcomes relate to overweight and obesity per se.
Introduction
The role of exercise in appetite control and weight management, as well as in the treatment of metabolic disorders such as type 2 diabetes (T2D), has been a prominent focus in numerous research studies. The phenomenon of exercise-induced anorexia was investigated and described by King, Burley and Blundell in 1994,1 who demonstrated that subjective appetite ratings were lower immediately following an acute bout of exercise compared with pre-exercise levels. Although absolute energy intake after the exercise was not suppressed, a long duration (∼50 min), high-intensity exercise bout led to a lower energy intake relative to the energy expended.1 Consistent findings have since shown that post-exercise energy intake, relative to the energy expenditure during exercise, is often reduced compared with resting condition. This indicates that exercise can create an energy deficit that is not fully compensated for by short-term increases in energy intake. However, the effects of exercise on absolute energy intake remain inconclusive, with reports of increased, decreased and no difference in energy intake compared with rest.2–4 These discrepancies may be attributed to methodological variations, including differences in exercise intensity, the timing of the post-exercise meal and the characteristics of the study population.
Circadian or diurnal rhythms may influence appetite control and consequently, energy balance. Exercise has been identified as a circadian time cue, with exercise-induced signals shown to affect molecular clock genes.5 The diurnal timing of exercise also seems to impact body weight, with greater losses in body weight observed with morning compared with evening exercise.6 7 However, research on the effects of exercise timing on appetite and energy intake has yielded mixed results. Some studies found no significant differences in energy intake at any meal or overall during 26 hours post-exercise or macronutrient preferences between morning and evening exercise.8 However, others reported that morning exercise may lead to greater perceived satiety 15 min post-exercise in women with overweight, although 24-hour post-exercise energy intake was similar.9 Exercise timing can also affect food preferences, with a greater wanting for low-fat sweet foods after morning compared with evening exercise and a greater wanting for high-fat sweet foods after evening compared with morning exercise.10 The impact of exercise timing may be influenced by individual chronotype, with early chronotypes experiencing greater hunger suppression after morning exercise and late chronotypes after evening exercise10; however, there is a need for a better understanding of the interaction between chronotype and exercise timing for appetite control and weight management.11
Furthermore, whether exercise affects appetite control differently in people living only with overweight/obesity or with overweight/obesity and T2D is currently unknown. In healthy individuals, glucose tolerance to identical meals is greater earlier compared with later in the day.12 However, in people with T2D, insulin sensitivity has been shown to improve throughout the day and worsen throughout the night into the morning.13 Whether this diurnal variation affects appetite control remains unclear. A few small studies in people at risk of or with T2D have investigated the effects of exercise timing on glycaemic control, with some suggesting that afternoon/evening exercise may lead to better glucose regulation compared with morning exercise.14–16 However, other studies have not observed significant differences.17 18 Additionally, people living with T2D are often prone to late-night eating,19 which could serve as a potential target for exercise interventions. If evening exercise could suppress appetite and reduce energy intake during the hours following the exercise bout, it may provide a viable strategy to mitigate late-night eating behaviour in this population.
Understanding ‘when’ to eat and exercise is of great importance as most clinical guidelines and interventions in the prevention and treatment of obesity and related diseases focus on ‘what’ and ‘how much’ to eat or ‘how’ and ‘how much’ to exercise. This study will investigate how exercise and exercise timing affect appetite control and subsequent energy intake after an acute, high-intensity exercise bout in people with overweight/obesity and with and without T2D.
Objectives
The specific objectives of the TIMEX study are to investigate whether energy intake during an ad libitum meal differs following a 45-min acute bout of high-intensity interval exercise compared with rest; investigate whether the energy intake of the ad libitum meal differs between morning and late afternoon; investigate if appetite ratings, food reward, metabolic markers following exercise and the ad libitum meal differ between morning and late afternoon, and between individuals with and without T2D; and investigate circulating biomarkers that can be used to identify if ad libitum food intake is primarily driven by hedonic or homeostatic appetite.
Hypotheses
Hypotheses for the primary endpoint (ad libitum energy intake) are:
An acute bout of exercise reduces ad libitum energy intake compared with a rest condition.
Time of day does not affect exercise-induced anorexia (ie, reduction in energy intake after exercise).
Hypotheses for the secondary endpoints are:
Appetite sensations and reward for high-fat sweet foods will be greater in the late afternoon relative to the morning and this effect will be more pronounced in individuals with T2D compared with individuals without T2D.
Appetite sensations and reward for high-fat sweet foods will be greater in response to exercise in the late afternoon relative to the morning and this effect will be more pronounced in individuals with T2D compared with individuals without T2D.
Appetite and food reward will be associated with circulating biomarkers and these associations will be affected by T2D status.
Methods and analysis
Study design
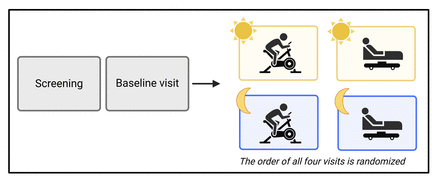
This study is a randomised, controlled, cross-over trial (figure 1). Fifty-eight individuals with overweight/obesity with or without T2D will be randomised after the completion of a screening and baseline visit. Each participant will complete four test visits (two morning visits and two late afternoon visits) on four separate days with a ≥3 day washout between visits. We aim for the completion of all visits within a 3-month period for each participant to prevent potential changes in, for example, body weight, dietary intake and physical activity. Primary and secondary outcomes are assessed during the baseline visit and test visits.
TIMEX study design. Baseline visits are completed after screening on the same day. Morning visits are initiated at 08:00–08:30 and late afternoon visits are initiated at 15:00–15:30. All visits are completed within 2.5 hours, and with a minimum of a 3-day washout.
The study will be conducted at Steno Diabetes Center Copenhagen (SDCC) and will be reported according to the Consolidated Standards of Reporting Trials.20 The study protocol follows the Standard Protocol Items: Recommendations for Interventional Trials statement.21 The study is registered at ClinicalTrials.gov (identifier: NCT05768958).
Participants
Women and men, 18–75 years, with overweight or obesity (body mass index (BMI) ≥25 kg/m2) with or without T2D, who are eligible according to the inclusion and exclusion criteria (box 1) will be included. We aim for an equal distribution of participants with and without T2D.
Eligibility criteria
Inclusion and exclusion criteria are listed in box 1.
Inclusion and exclusion criteria for participation in the TIMEX study
Inclusion criteria
Adults with overweight or obesity (BMI >25 kg/m2) with and without T2D.
Age 18–75 years.
HbA1c ≥48 mmol/mol for people with T2D.
Waist-to-height ratio ≥0.5 or waist circumference ≥88/102 cm for women and men, respectively.
Exclusion criteria
Not able to eat the ad libitum meal because of, eg, allergy or intolerance.
Not able to perform the exercise bout because of, eg, musculoskeletal conditions.
Daily smoking.
For women: pregnancy/planned pregnancy (within the study period)/lactating.
Self-reported history of an eating disorder in the past 3 years.
Self-reported weight change (>5 kg) within 3 months prior to inclusion.
Treatment with antidepressants.
Treatment with fast-acting insulin, combination insulin products and sulfonylureas.
Alcohol/drug abuse or in treatment with disulfiram (Antabuse) at the time of inclusion.
Uncontrolled medical issues including but not limited to cardiovascular, pulmonary, rheumatological, haematological, oncological, infectious, gastrointestinal or psychiatric disease; diabetes or other endocrine disease; immunosuppression.
Current treatment with medication that significantly affects appetite or energy balance (eg, glucagon-like-peptide 1 receptor agonists).
Bariatric surgery.
Unable to understand the informed consent and the study procedures.
Concomitant participation in intervention studies.
Incapable of understanding Danish.
BMI, body mass index; HbA1c, glycated haemoglobin; T2D, type 2 diabetes.
Outcomes
Primary outcome
The primary outcome is the difference in energy intake during an ad libitum meal after 45 min of acute exercise bout compared with rest. The ad libitum meal will be served 15 min post-exercise.
Secondary exploratory outcomes
All secondary outcomes include a comparison between exercise and rest, morning and late afternoon and T2D status. Secondary outcomes include eating rate; 24-hour energy intake (comprised of the energy intake from the ad libitum meal and self-reported energy intake 24 hours post-visit); appetite-related metabolites and hormones, and circulating biomarkers assessed from proteomics, metabolomics and lipidomics analyses; food choice, food attention and reaction time, explicit and implicit liking and wanting, subjective appetite; ratings of perceived exertion during exercise.
Recruitment
Screening/baseline visit
Participants are recruited via advertisements on www.Forskningnu.dk (social media). After reading the study information, they undergo a prescreening phone interview to reduce screening failures. During the screening visit, participants are orally briefed on the study, provide written consent (online supplemental material) and undergo a health examination, including medical history and eligibility assessment (box 1). If eligible, participants proceed with height, weight, blood pressure and ECG measurements. Those with approved ECGs complete a bicycle test to determine peak oxygen uptake (VO2peak). After inclusion, participants are randomised to their test visit sequence, which are scheduled to reduce confounding variables.
Supplemental material
Anthropometry and blood pressure
At the baseline visit, body weight will be measured to the nearest 0.1 kg with the participant wearing light clothes. Height will be measured to the nearest 0.1 cm. Height and weight will be measured using Seca 287 wireless ultrasonic measuring station (Seca gmbh & co. kg, Hamburg, Germany). Blood pressure and resting heart rate will be measured using a digital blood pressure gauge (Microlife BP A3L Comfort, Microlife AG Swiss Corporation, Widnau, Switzerland) and noted three times with 2-min intervals after a 10-min rest and the average of the two lowest values is reported.
Cardiorespiratory fitness
VO2peak as a measure of cardiorespiratory fitness will be measured by an incremental bicycle ergometer test (Corival CPET Medical Ergometer, Lode BV, Groningen, The Netherlands). Participants will complete a warm-up of 3 min at 25 watts followed by a linear increase in watts until exhaustion. Heart rate, ventilation rate, inspired oxygen and expired carbon dioxide levels are measured using a combined heart rate monitor and indirect calorimetry (Vyntus CPX, Vyaire Medical, Hoechberg, Germany). The VO2peak test will be deemed valid if the respiratory exchange ratio is >1.15, a plateau in oxygen uptake is reached and the heart rate is ±10 heartbeats from the estimated maximal heart rate.22
Test visits
All test visits will be conducted at SDCC. Prior to their visit, the participants are asked to standardise sleep (7–9 hours) if possible and dietary intake the day before all test visits. Participants will be asked to register their dietary intake 24 hours prior to and after each test visit. To obtain a somewhat equal baseline, the participants will further be asked to follow the same dietary pattern (to the best of their ability) during the 24 hours before each visit. On test days, participants will arrive at ∼8:00 after an ≥8 hour fast (morning visits) or at ∼15:00 after a 3-hour fast (late afternoon visits). Water is allowed until 2 hours before each visit. Smoking is allowed until 3 hours before each visit. Furthermore, no alcohol consumption or strenuous physical activity is allowed 48 hours prior to testing. Participants are instructed to avoid physically demanding transportation to the research facility. Participants are instructed to continue their regular medication regimen throughout the duration of the study. The schedule for the test visits is represented in figure 2, and a schematic overview is presented in table 1.
Schedule for the test visits. SBFPT, Steno Biometric Food Preference Task; VAS, Visual Analogue Scales.
Schematic overview of measurements during study visits
Blood samples
Blood samples will be collected from an antecubital vein in the fasted state on the test visits and at four subsequent time points (0, 15, 45 and 60 min after the completion of the exercise bout/rest). Table 2 presents the analyses that will be performed.
Schematic overview of blood sample measures
Body composition
Body composition, that is, fat mass and fat-free mass, will be examined by electrical bioimpedance (seca mBCA 515, Seca, Hamburg, Germany) at the first morning test visit with the participant wearing light clothes.
Dietary intake
Participants will be instructed to weigh and register their food and drink intake 24 hours before and after each test visit. For the registration, each participant receives a printed food diary containing written instructions for tracking, and tables for the tracking. All participants are offered to borrow a digital food scale. The hand-written, self-reported dietary intake will subsequently be logged into the online software Vitakost.dk (Vitakost, Kolding, Denmark) by the researchers.
Questionnaires
Participants will complete questionnaires on:
Socioeconomic status (SES).23
Eating behaviour (Control of Eating Questionnaire—CoEQ).24
Physical activity (International Physical Activity Questionnaire—IPAQ).25
Chronotype (Morningness/Eveningness Questionnaire—MEQ26 and Munich Chronotype Questionnaire—MCTQ.27
Sleep (Pittsburgh Sleep Quality Index—PSQI).28
Appetite and meal assessment (Visual Analogue Scales—VAS).29
The participants will fill in the questionnaire CoEQ at the baseline visit, and the remaining (SES, IPAQ, MEQ, MCTQ and PSQI) at their first rest visit.
Subjective appetite
Subjective appetite will be assessed using VAS,29 including ratings of hunger, fullness, satiety and prospective food consumption, thirst, desire to eat something sweet, salty, fatty, meat and potential nausea. We will collect the VAS measurements in the fasted state on the test visits and at five subsequent time points (0, 15, 30, 45 and 60 min after the completion of the exercise bout/rest).
Palatability and liking of the ad libitum test meal will be rated. The ratings will be performed on a computer during all test visits.
Socioeconomic status
Participants’ SES will be assessed using the SES questionnaire23 performed on a computer during the first rest visit. The SES questionnaire includes information on gender, educational level, work position, family orientation and annual household income.
Eating behaviour traits
Participants’ eating behaviour traits will be assessed using CoEQ24 performed on a computer during the baseline visit. The CoEQ includes questions on hunger, fullness, cravings and mood over the past 7 days.
Physical activity
Participants’ physical activity will be assessed using IPAQ25 performed on a computer during the first rest visit. The IPAQ includes questions on frequency and intensity of physical activity on average over the past 7 days.
Chronotypical features
Data on participants’ chronotypical features will be assessed using MEQ and MCQT.26 27 The questionnaire will be performed on a computer during the first rest visit. It will include questions on subjective fatigue, awakeness, energy level and hunger during mornings and nights.
Food preferences
Food preferences will be measured using the diurnally validated SBFPT,30 31 a computerised task adapted from the Leeds Food Preference Questionnaire and tailored to fit a Danish food context.32 The task includes 16 images of common Danish foods, varying in fat content (high or low) and taste (sweet or savoury). Before the study, an online survey validated the appropriateness of these images for morning and late afternoon consumption among the target population (n=167).31 The SBFPT has two parts: (1) a forced-choice task to measure food choice and implicit ‘wanting’, and (2) a rating task to assess explicit ‘liking’ and explicit ‘wanting’ on a scale from 0 to 100.
All responses are recorded in the digital software, iMotions 9.3 (iMotions A/S, Copenhagen K, Denmark) and used to compute mean scores for all the abovementioned combined food categories. Prior to these two parts, participants are asked to familiarise themselves with a printed version of the 16 food images and explain what they see in the images. During the presentation of the 16 pictures, data on eye tracking and reaction time are collected. Eye tracking will be used to examine visual attention to food items through analyses of, for example, gaze duration bias and gaze direction bias. Eye tracking will be examined by tracking participants’ eyes on the computer screen using the Tobii X2-60 device (Tobiipro, Stockholm, Sweden) integrated into the iMotions software.
Exercise bout
The workload of the acute exercise bout will be individually calculated based on the peak workload (Wattpeak) achieved at the VO2peak test. The total duration of the exercise bout is 45 min divided into intervals: 10 min warm-up (40% Wattpeak); 4×4 min high intensity (75% Wattpeak) with 3 min active rest (50% Wattpeak) between intervals; 10 min cool-down (40% Wattpeak). Initially, the high-intensity intervals were set at 85% Wattpeak, however, after four participants we changed the workload to 75% Wattpeak since participants were not able to complete the exercise at the 85% Wattpeak. In situations where a participant is not capable of completing the precalculated workload, the load will be manually downregulated to ensure a 45-min exercise bout. During the exercise bout, heart rate, workload and revolutions per minute (rpm) are measured continuously. Participants will be asked to rate their perceived exertion (using the Borg Scale) at the end of the warm-up, the end of the last low-intensity and high-intensity interval, and at the end of the cool-down period. Female participants are given 200 mL water and male participants are given 250 mL water during the 45-min exercise bout and asked to finish the water before completion. On the rest test days, participants will rest for the same duration as the exercise bout.
Ad libitum meal
Participants will be served an ad libitum meal 15 min after the termination of the exercise bout/rest. The meal consists of savoury pie, from which each participant can choose between three different types: bacon, leek, or pesto and tomato. The nutritional value of the different pies is presented in table 3. The participants will be presented with two pies of 800 g in total and instructed to eat until comfortably full/satiated. Participants will be presented with the same type of pie at all visits. The pies will be divided into randomly sized portions to minimise the likelihood of participants estimating their consumption based on previous visits. The portions will be served on a large plate for consistency in presentation. Female and male participants receive 200 mL and 250 mL water, respectively, during the ad libitum meal, and are asked to finish the water before completing the meal. After completion of the meal, the remaining portion of the pie will be measured, and the total weight of the consumed pie will be calculated.
Nutritional value of ad libitum meal options
Statistical methods
Sample size
The study was designed to detect a minimally important difference (MID) of 500 KJ33 with an alpha of 0.05 and a beta of at least 0.8 for testing the hypotheses for the primary outcome in a hierarchal manner. Based on information from the literature2 21 34–36 and observed data from similar ad libitum meal tests performed in one of our previous studies,37 we produced simulated data to use for the sample size calculation of the study. The primary scenario assessed was: acute exercise reduces ad libitum intake by 1 MID both in the morning and in the late afternoon. This scenario was used to test hypothesis 1 (superiority of exercise vs rest for reducing ad libitum intake) and hypothesis 2 (equivalence of exercise in the morning vs exercise in the late afternoon). The simulated data were analysed using a repeated measures regression model (mixed linear model, PROC MIXED, SAS V.9.4, SAS Institutes Inc) specified as: ad libitum intake~sex + condition (morning/rest, morning/exercise, late afternoon/rest, late afternoon/exercise) + sequence (1–4), a repeat for condition on participant level, an unstructured covariance structure and alpha=0.05. A total of 53 participants will be needed to complete the study to attain the desired statistical power. To account for potential loss-to-follow-up an additional 5 (10%) participants will be recruited. Participants who withdraw after randomisation, but before completing any test visits will be replaced.
Statistical analysis plan
All outcomes will be reported as a summary of the raw data using appropriate summary statistics. Descriptive data are presented as mean±SD if normally distributed, as median (IQR) if non-normally distributed and as n (%) if categorical.
Outcomes measured on the test days will as a general rule be analysed using a linear mixed model (LMM). LMMs will be used to assess energy intake differences across conditions (rest vs exercise), time of day (morning vs late afternoon), and their interactions, as well as the randomised visit sequence, while accounting for within-subject variability with a random intercept on participant level. We will calculate levels for each condition and the contrasts between them (estimated levels and differences with 95% confidence intervals (95%CI) will be calculated and presented). The interaction with T2D status on the outcomes will also be explored using T2D as an interaction term in the model. In the LMM with T2D interaction, we will adjust for age, sex and BMI to account for potential differences between groups.
Model assumptions will be assessed using graphical methods, including Q–Q plots, residual versus predicted plots, and histograms of residuals. If needed outcomes will be log-transformed for analysis and results back-transformed for presentation. Outcomes that do not fit the model will be analysed using a generalised mixed model or by comparing the observed data using non-parametric null-hypothesis tests.
Modelled outcomes will be presented as estimated levels (95% CI) on test days and comparisons between test days will be presented as estimated differences (95% CI, p values).
P values <0.05 will be regarded as statistically significant. The false-positive rate related to the hypothesis for the primary outcome will be controlled by using a hierarchal testing procedure. Secondary/descriptive outcomes will not be adjusted for multiplicity, apart from omics outcomes (proteomics, metabolomics and lipidomics) where a false discovery rate cutoff (<0.1) will be applied.
Laboratory analysis
Glucose, insulin, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, alanine aminotransferase, aspartate transaminase, natrium, kalium, C-reactive protein and glycated haemoglobin analyses will be performed immediately at the Department of Clinical Biochemistry, Herlev Hospital, Denmark (adjacent to SDCC).
One blood sample (3 mL) will be treated with dipeptidyl peptidase 4-inhibitor ‘ValPyr’ at the time of collection. Blood samples not immediately analysed will be centrifuged at 4°C and 3000 rpm for 10 min. Plasma will then be transferred to cryotubes and stored at −20°C while the test visit is ongoing and then immediately transferred to a −80°C freezer for storage after the visit. All other analyses will be performed after study completion in thawed samples in collaboration with the specific laboratory of excellence. The hormones scheduled for analysis following study completion include: ghrelin, glucagon, gastric inhibitory polypeptide, glucagon-like-peptide 1, peptide tyrosine tyrosine, pancreatic polypeptide, cholecystokinin, fibroblast growth factor 21 and growth differentiation factor 15.
Omics analysis
The proteomics analysis, liquid chromatographic-mass spectrometry, will be performed at Novo Nordisk A/S.
Metabolomics analysis (gas chromatographic-mass spectrometry), and lipidomics analysis (ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry) will be performed at SDCC.
Ethics and dissemination
Ethics
The knowledge obtained from this study will contribute to a better understanding of appetite control in response to acute exercise and timing of exercise in people with overweight/obesity, and people with overweight/obesity and T2D. The study has been approved by the Ethics Committee of the Capital Region of Denmark (H-22019913) and the Capital Region of Denmark’s Research Register (Privacy). The study will be conducted in accordance with the Declaration of Helsinki. The study has been registered at ClinicalTrials.gov (NCT05768958). Participants will provide informed consent orally and in writing. All study results (positive, negative and inconclusive) will be published in international peer-reviewed scientific journals and presented at national and international conferences.
Reporting patient and public involvement in research
Individuals in the target group were involved in the initial stages of the TIMEX study. Their involvement covered two parts of the study:
To ensure a diurnally validated version of the SBFPT that would allow us to examine food reward both in the morning and late afternoon using the same food images, we conducted an online questionnaire containing 28 food items (see Test Visits; Steno Biometric Food Preference Task).31 The details are elaborated in a study by Beaulieu et al.31 Based on the answers, we selected 16 items to be included in the SBFPT. This involvement from participants in our target group ensured a diurnally validated version of the food images and thus a higher quality of the SBPFT.
Individuals within our target group actively participated in selecting an appropriate ad libitum meal for the test visits. The process included three phases: The first phase involved SDCC employees engaged in the TIMEX study and members of our research group. Together we compiled a list of meals suitable for consumption in the morning and late afternoon, with high calorie density and high fat content. The second phase involved colleagues and members of our target group. Insights from individuals with T2D helped us clarify a meal balancing low carbohydrate content with high caloric density. The third phase involved meal testing in our laboratory to ensure consistent preparation and ease of disassembly. Experience from the laboratory resulted in a test meal choice of savoury pie. We opted to provide participants with a selection of three different pies to account for individual taste preferences, allergies and dietary restrictions (eg, religious considerations).
Incorporating the target group into these considerations proved highly beneficial. The insights provided by participants with T2D were particularly valuable. Participants with T2D exhibited a reluctance toward consuming foods high in carbohydrates compared with those without T2D. Without their involvement, there was a risk of selecting a meal that would not align with the preferences of people with T2D, potentially hindering recruitment for the study.
Discussion
Despite current clinical guidelines and interventions to prevent and treat obesity, the global prevalence of obesity continues to increase. Additionally, there is a growing number of individuals living with T2D. Guidelines focusing on ‘what’ and ‘how much’ to eat or exercise are unmanageable for many people and are too time-consuming, explaining the need for more feasible strategies. An increasing focus on hedonic mechanisms, diurnal rhythms and food reward systems indicate differences between lean individuals and individuals with overweight/obesity and/or T2D. This novel research raises important questions. Does acute exercise affect appetite in people with overweight, obesity and T2D and is the effect influenced by the timing of exercise? If physical activity can influence hedonic aspects of appetite control, does exercise timing affect this? Does obesity with or without T2D alter food reward? Building on these inquiries, the TIMEX study aims to investigate whether or to what extent acute exercise and timing of exercise can affect appetite control in individuals with overweight/obesity with and without T2D.
The cross-over study design enables comparison of appetite ratings, metabolic response and subsequent food intake following an acute exercise bout compared with a rest condition where each participant serves as their own control. Furthermore, how this might differ between morning and late afternoon, and between individuals with obesity with or without T2D will also be examined. Blood samples taken during each test visit will reveal how appetite-related metabolites, hormones and other biomarkers are influenced by the timing of exercise, and how these effects differ between weight and diabetes status.
Ethics statements
Patient consent for publication
References
Footnotes
X @J_Salling_Quist
LJ and NPL contributed equally.
Contributors Conceptualisation: MKG, GF, KB, KF and JSQ. Methodology: NPL, HEP, MBB, GF, KB, KF, LGG and JSQ. Software: NPL, MMJ, HEP and MBB. Investigation: LJ, NPL, MMJ, HEP and MBB. Writing—original draft: LJ and NPL. Writing—review and editing: LJ, NPL, MMJ, HEP, MBB, MKG, GF, KB, KF, LGG and JSQ. Visualisation: LJ and NPL. Supervision: GF, KB, KF, LGG and JSQ. Project administration: LJ, NPL, HEP and MMJ. Funding acquisition: MKG, GF, KB, KF, LGG and JSQ. Guarantor: JSQ.
Funding The study is initiated by the investigator and co-investigators at Steno Diabetes Center Copenhagen ( SDCC). The total budget of the study is DKK 2 970 400 and will cover expenses for laboratory analyses, salaries, some equipment and operating expenses. The study is funded by a NovoSTAR grant from Novo Nordisk A/S (DKK 2 970 400) granted investigator, JSQ. The grant is paid to and handled by SDCC. If needed or if possible, additional funding will be sought from relevant partners and foundations. The Ethics Committee of the Capital Region of Denmark and the study participants will be informed of the grant source and the amount, if additional funding is obtained. Study participants receive DKK 400 for each study visit completed (excluding the screening visit). The remuneration is compensation for the inconvenience associated with participation in the study. Travel expenses are covered if individuals live more than 10 km away with a maximum of DKK 500 per visit. This applies to both use of public transportation and private transportation by car (car expenses of DKK 2.16 per km). Transportation by car will be registered as a transport subsidy. There is no treatment gain for the participants in the study. Participants who withdraw or are excluded from the study will receive remuneration corresponding to the number of study visits completed. Participants will not receive other benefits of economic value.
Competing interests LJ, NPL, MMJ, MBB, LGG and JSQ are employed by Steno Diabetes Center Copenhagen ( SDCC). SDCC is a hospital providing health services for the public health care system. SDCC is funded by the Novo Nordisk Foundation through unrestricted grants. The Novo Nordisk Foundation has no economic interests in the study. The Novo Nordisk Foundation will not have influence on (1) the study design; (2) the collection, analysis and interpretation of data; (3) the writing of the study report or any publication; and (4) the decision to submit the paper for publication. The investigators employed at SDCC will not benefit economically from conducting the study. Co-investigator MKG is employed at Novo Nordisk and will be responsible for exploratory investigations using the data collected in the TIMEX study. Due to the collaborative nature of the study, Novo Nordisk has been involved in designing the study and will be involved in analysing and interpreting data. Novo Nordisk will align with SDCC if and when data and/or biosamples from the study are used for publications. HEP and KF are now employed by Novo Nordisk. iMotions is a collaborator on the project and give advice for the use and analysis of biometric methods in the study design phase. iMotions A/S have no influence on (1) the study design; (2) the collection, analysis and interpretation of data; (3) the writing of the study report or any publication; and (4) the decision to submit the paper for publication. LGG and KF own shares in Novo Nordisk. The sponsor and principal investigator, JSQ, have no economic interest in the results of the study. All other authors have no competing interests to declare.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.