Article Text
Abstract
Introduction The prognosis for epithelial ovarian cancer (EOC) is exceedingly poor, with patients diagnosed with stage III/IV tumours typically offered cytoreductive surgery in conjunction with chemotherapy as a standard treatment option. This approach is intended to reduce the risk of surgery and address ovarian cancers that are not amenable to surgical intervention. A promising alternative and important treatment option is neoadjuvant chemotherapy (NACT) in conjunction with interstitial tumour cytoreductive surgery. The combination of neoadjuvant immunotherapy with chemotherapy has recently demonstrated remarkable efficacy, particularly in melanoma and lung cancer, with notable pathological responses and therapeutic benefits in tumour tissue. The NeoAdjuvant chemotherapy with or without tIslelizumab followed by debulking surgery for oVarian cancEr(NAIVE) study aims to assess the clinical efficacy and safety of NACT in combination with tislelizumab (a monoclonal antibody for programmed cell death protein 1) for advanced EOC.
Methods and analysis The NAIVE study is an investigator-initiated, prospective, single-centre, open-label, randomised controlled trial for advanced EOC with the International Federation of Gynaecology and Obstetrics (FIGO) stage IIIc with a Suidan CT score of 3 or greater or a Fagotti laparoscopic score of 8 or greater; or FIGO stage IV. The primary endpoint of the study is the 1-year progression-free survival (PFS) rate, measured as the percentage of patients who are free of tumour progression or death for 1 year after receiving the first dose of study drug. The secondary endpoints encompassed the R0 resection rate, the clinical response rate and other relevant metrics. Enrolled patients will be randomly assigned in a 1:1 ratio to either the experimental arm, which will receive neoadjuvant platinum-based chemotherapy in combination with tislelizumab, or the control arm, which will receive neoadjuvant platinum-based chemotherapy. The study will enrol 40 patients, with enrolment scheduled to start in April 2021 and complete in April 2025, given a 1-year PFS rate of 60%. The study will provide new evidence regarding the clinical efficacy and safety of NACT in combination with tislelizumab for advanced ovarian cancer. The results will contribute to a deeper understanding of the clinical effects, safety profile and fundamental immunological processes. The findings will contribute to the growing body of evidence in support of the incorporation of immunotherapy into the treatment paradigm for ovarian cancer, thus facilitating the development of more personalised and efficacious therapeutic modalities.
Ethics and dissemination This trial has received ethical approval from the Institutional Ethics Committee of the Second Affiliated Hospital of the Medical College of Zhejiang University. Presentations at scientific and professional meetings and publication in peer-reviewed journals will disseminate the results of the study.
Trial registration number NCT04815408.
- Randomised Controlled Trial
- ONCOLOGY
- CHEMOTHERAPY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is a prospective, randomised, controlled clinical trial designed to evaluate the efficacy and safety of neoadjuvant chemoimmunotherapy in ovarian cancer.
Tumour tissue biopsies will be collected for the purpose of a translational research programme.
The utilisation of nab-paclitaxel in the formulation of chemotherapy protocols has been shown to obviate the immunosuppressive consequences of dexamethasone pretreatment.
Lack of blinding of interventions.
The study is being designed and conducted in a hospital of China, limiting the generalisability of results for other settings and people with advanced ovarian cancer.
Introduction
Ovarian cancer represents a significant health concern for women on a global scale, with an incidence and mortality ranking of eighth and seventh, respectively, among female cancers worldwide. The insidious nature of the disease, in addition to the absence of effective screening programmes, results in the majority of patients being diagnosed with advanced-stage disease, including the International Federation of Gynaecology and Obstetrics (FIGO) stages III and IV.1 Primary debulking surgery (PDS) followed by platinum-paclitaxel-based chemotherapy is the current standard of care for advanced ovarian cancer. Numerous studies have demonstrated that achieving complete cytoreduction during PDS has a significant impact on survival rates.2 Nonetheless, it is probable that a primary maximal surgical endeavour will engender an elevated occurrence of perioperative surgical complications and severe trauma, thereby giving rise to concerns regarding the optimal balance between efficacy and safety.3
As an alternative treatment strategy, neoadjuvant chemotherapy (NACT) with interval debulking surgery (IDS) has been proposed.4 A review of the literature indicates that survival after NACT followed by IDS is comparable to that after PDS followed by chemotherapy.5 In the National Comprehensive Cancer Network (NCCN) guidelines for ovarian cancer, neoadjuvant therapy is defined as a treatment regimen administered prior to surgical resection of the tumour to reduce its size. For stage IIIC and IV ovarian cancer, NACT followed by IDS is a standard form of alternative therapy in the medical community.6 There is an urgent need to investigate new effective therapeutic modalities that can control the disease preoperatively, facilitate complete cytoreduction and improve overall survival (OS).
In recent years, immune checkpoint inhibitors (ICIs), including those targeting programmed cell death protein 1 (PD-1)/PD-L1, have emerged as a highly effective and promising therapeutic approach for a variety of malignancies.7 Although retrospective studies have indicated the possibility of enhanced survival with ICIs in ovarian cancer, prospective clinical trials have not substantiated the efficacy of ICIs as monotherapy or combination therapy in epithelial ovarian cancer (EOC).8 The utilisation of neoadjuvant ICIs has been evidenced to elicit substantial pathological responses across a spectrum of cancer types, including melanoma, lung cancer, colorectal cancer and bladder cancer.9–11 In the context of neoadjuvant immunotherapy, the therapy exerts an immune-killing and surveillance effect based on the presence of a tumour by inhibiting the immune checkpoint pathway and significantly stimulating the T cells infiltrating the tumour. It is hypothesised that the intact tumour immune microenvironment is conducive to the most potent response to ICIs.12 Consequently, neoadjuvant immunochemotherapy represents a novel treatment option for advanced ovarian cancer.
In this scenario, a novel alternative treatment strategy, namely NACT in conjunction with delayed surgery, has the potential to enhance the rate of surgical resection, reduce the incidence of surgery-related complications, improve survival outcomes and optimise quality of life. Tislelizumab, a humanised IgG4 monoclonal antibody, has been engineered to bind with high affinity and specificity to PD-1. The antibody has been engineered to minimise binding to Fc-gamma receptors on macrophages, thereby facilitating tumour recognition and elimination by the body’s immune cells. The efficacy of this combination, in particular its antitumour efficacy, has been demonstrated in the treatment of multiple advanced tumours. Furthermore, the combination has exhibited an acceptable safety and tolerability profile.13 The objective of this prospective clinical trial is to assess the clinical efficacy and safety of NACT in combination with tislelizumab for advanced EOC. Furthermore, the study will examine the dynamics of the immune microenvironment within the tumour tissue during combination therapy and the relationship between these changes and efficacy.
Methods and analysis
Design
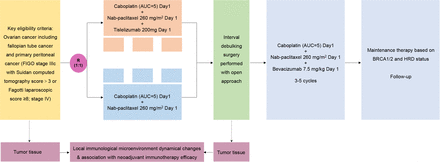
The NeoAdjuvant chemotherapy with or without tIslelizumab followed by debulking surgery for oVarian cancEr (NAIVE) study is a prospective, phase II, single-centre, open-label, randomised controlled trial employing a parallel design. Subjects will be randomly allocated in a 1:1 ratio to either the experimental arm, which will receive neoadjuvant platinum-based chemotherapy in combination with tislelizumab, or the control arm, which will receive neoadjuvant platinum-based chemotherapy. This protocol is reported in accordance with the guidelines for the reporting of randomised controlled trials (SPIRIT) 2013 statement. Figure 1 and table 1 illustrate the NAIVE schematic and study schedule, respectively. The patient registration process was conducted between May 2021 and May 2025. The 5-year follow-up is designed for all patients, and the final study report will be prepared within 6 months. Consequently, the study is scheduled to conclude in October 2030.
Study schematic. AUC, area under curve; FIGO, International Federation of Gynaecology and Obstetrics; HRD, Homologous Recombination Deficiency.
Schedule for enrolment, interventions and assessments (SPIRIT figure)
Objective of the study
This study is designed to evaluate the efficacy and safety of neoadjuvant platinum-based chemotherapy combined with tislelizumab in patients with FIGO stage IIIc–IV EOC. The patients will be evaluated for 1-year progression-free survival (PFS), tumour clinical response to treatment and pathological response, surgical outcome (percentage of R0 resections) and OS. Furthermore, the incidence and severity of treatment-related toxicity will be evaluated. Furthermore, this study will assess the dynamic evaluation of the local immune microenvironment in the primary foci of ovarian cancer and its correlation with the efficacy of neoadjuvant immunotherapy.
Endpoints of the study
The primary endpoint of this study is the 1-year PFS rate, measured as the percentage of patients who are free of tumour progression or death for 1 year after receiving the first dose of study drug. PFS is the time from tislelizumab to disease progression or death, per RECIST V.1.1.
The secondary endpoints included:
R0 resection rate (R0%): This is defined as the proportion of patients who have undergone complete resection following interval cytoreductive surgery.
Clinical response rate (cRR): This is the proportion of patients who achieve a clinical complete or partial response. The cRR is assessed by two blinded independent investigators according to CT/MRI by RECIST V.1.1.
Pathological response rate (pRR): This is calculated as the proportion of patients who achieve a pathological complete or partial response. Pathological assessment was performed by two blinded independent reviewers using a three-tiered Chemotherapy Response Score (CRS) system.
OS: Determined from the date of enrolment to the date of death from any cause or the last follow-up visit.
Adverse events (AEs): AEs were reported according to the Common Terminology Criteria for Adverse Events, V.5.0 (CTCAE V.5.0).
Participants and setting
Participants will be recruited from a single centre: the Department of Gynecology, the Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou, China. The recruitment period will span from May 2021 to May 2025. Patients who have been newly diagnosed with the condition and who have been deemed suitable for neoadjuvant treatment by a physician during the initial evaluation will be considered for inclusion in the study. The inclusion and exclusion criteria are delineated in box 1. All eligible patients at the study sites will be recruited directly in the clinic. Prior to any trial-related procedures being performed, patients must be thoroughly informed about the study in an undisturbed room, and they must sign and date the informed consent form. Patients are offered a minimum of 24 hours to consider their participation in the study. Participation in the study will not influence future treatment, and patients may withdraw from the trial at any time without providing a reason or being subject to any prejudice regarding further treatment.
Inclusion and exclusion criteria
Inclusion criteria
Individuals who meet the following criteria will be included in the study.
Individuals between the ages of 18 and 75 years.
Pathologically confirmed as epithelial ovarian cancer, fallopian tube cancer or peritoneal cancer (exclusive of mucinous adenocarcinoma).
International Federation of Gynaecology and Obstetrics (FIGO) stage IIIc with a Suidan CT score of 3 or greater or a Fagotti laparoscopic score of 8 or greater; or FIGO stage IV.
Eastern Cooperative Oncology Group performance status of 0–2, with the ability to tolerate chemotherapy.
No previous immunotherapy for malignant tumours.
The subject must demonstrate an ability to comprehend the nature of the study and must have signed the informed consent form.
Exclusion criteria
Subjects who meet the following criteria will be excluded from the study:
Concomitant with other uncontrolled malignancies.
Any disease requiring systemic treatment with corticosteroids (daily dose of prednisone or equivalent >10 mg) or other immunosuppressive drugs within 14 days before randomisation. The use of local or alternative corticosteroids (daily dose ≤10 mg of prednisone or equivalent) is allowed, as well as short-term (≤7 days) prescription corticosteroids for prophylactic purposes or for treating non-autoimmune conditions. However, individuals with any active autoimmune disease or a history of autoimmune disease are excluded.
History of active autoimmune disease or autoimmune disease with a potential for recurrence. Controlled type 1 diabetes, hypothyroidism requiring only hormone replacement therapy, well-controlled coeliac disease, skin conditions that do not require systemic treatment (such as vitiligo, psoriasis or alopecia) or diseases expected not to relapse without external factors are allowed.
History of interstitial lung disease, non-infectious pneumonia or poorly controlled conditions (including pulmonary fibrosis, acute lung disease, etc).
Subjects with active hepatitis B (defined as a positive hepatitis B surface antigen test and HBV-DNA levels exceeding the upper limit of normal for the testing laboratory) or hepatitis C (defined as a positive hepatitis C surface antibody test and positive HCV-RNA) are excluded.
Known HIV infection (known to be HIV-positive).
Received a live vaccine within 30 days before the first dose, including but not limited to mumps, rubella, measles, varicella/zoster (chickenpox), yellow fever, rabies, Bacillus Calmette-Guérin and typhoid vaccine (inactivated viral vaccines are allowed).
Uncontrolled cardiac clinical symptoms or diseases.
Allergic to any of the drugs in this protocol.
According to the investigator’s judgement, subjects with any disease, treatment or laboratory abnormality that may confound the study results, interfere with the subject’s full participation in the study or is not in the best interest of the subject to participate.
Treatment
The regimens for patients enrolled in the NACT with the tislelizumab cohort are as follows: nab-paclitaxel 260 mg/m², carboplatin area under curve (AUC)=5 and tislelizumab 200 mg, to be administered intravenously. For patients in the NACT cohort, the regimen consists of nab-paclitaxel 260 mg/m² and carboplatin AUC=5, administered intravenously. Neoadjuvant therapy is administered every 3 weeks, with a total of three cycles. Post surgically, patients will receive therapy in accordance with the NCCN guidelines. AEs of grades 3–4 require the immediate cessation of treatment and active management to ensure resolution at grades 1–2. In the case of serious aAEs, the dose of carboplatin and albumin-bound paclitaxel may require reduction. In instances where immune-related aAEs do not return to grade 2 or lower before commencing the second cycle of combination therapy, it is recommended to discontinue tirellizumab.
Data collection
Prior to the initiation of screening evaluations, each participant will be furnished with the requisite information and afforded the opportunity to consent to their participation. Baseline data will be collected at the screening visit and completed within 14 days prior to enrolment. The clinical and laboratory assessment encompasses tumour size, quality of life, survival duration and haematological and biochemical parameters. The baseline data collection will include the subject’s gender, age, contact details, comprehensive medical history, medication history and drug allergy history. In addition, the Eastern Cooperative Oncology Group performance status, body mass index, complete blood cell count and liver and kidney function tests will be documented. Furthermore, data will be gathered at the baseline stage, during the neoadjuvant immunotherapy phase, at the time of surgery and during the postoperative adjuvant therapy phase. A baseline CT/MRI scan must be performed within 14 days prior to the commencement of treatment. The second CT/MRI scan must be conducted before surgery. The baseline and second assessments must adhere to the same radiological procedures, which include chest CT and abdomen CT/MRI. The assessment of treatment response will be conducted using RECIST V.1.1. Table 2 provides a comprehensive overview of the data collection process.
Data collection procedures
The degree of treatment toxicity will be measured by the incidence and severity of adverse effects, as evaluated at each clinic visit. AEs will be classified in accordance with the revised CTCAE V.5.0. In the event of a subject withdrawing from the study, the reason for treatment interruption, the date of discontinuation, the date and reason for withdrawal and the cause of death, if applicable, must be recorded. In the event that a subject is unable to continue with the investigation due to factors unrelated to disease progression, for example, adverse effects, violation of the trial protocol, withdrawal of informed consent or self-withdrawal, it is necessary to continue to monitor the patient until either disease progression or death occurs.
Data monitoring
The detailed data obtained from the patients will be recorded in the case report form (CRF). A central database will be responsible for the collection and management of said data. The maintenance and storage of all study records and original documents will be undertaken in accordance with the relevant regulations and guidelines, or by the research institution’s rules. The investigator accessing the relevant raw data of the clinical study is responsible for reviewing the CRF to determine the information’s completeness, accuracy and consistency with the source data. A central monitoring group, comprising trained and qualified individuals, will periodically visit each participating centre throughout the trial. The central monitoring group will ensure data submission, patient eligibility and protocol compliance.
Sample size and randomisation
This is an exploratory biomarker study, and any statistical comparisons between the two treatment arms will be exploratory in nature. Moreover, the study is structured as a parallel-group randomised trial, with the primary outcome being the 1-year PFS rate. The randomisation ratio is 1:1. Assuming a 1-year PFS rate of 60% in the control group14 15 and 80% rate (a 20% increase) in the treatment group, with a one-sided significance level of α=0.05 and 80% power, the study requires a sample size of 40 subjects (20 in each group), a dropout rate of 10% is assumed. The requisite sample size was calculated using PASS 15. Randomisation will be performed using a computer-generated programme that incorporates a random element, which will ensure a balance of the tumour stage.
Data analyses
An intention-to-treat analysis will be performed to evaluate the baseline characteristics and efficacy. A χ2 test will be employed to ascertain whether there are significant differences in the 1-year PFS rate, cRR, pRR and residual tumour rate (R0%) between the NACT plus tislelizumab and NACT groups. The Kaplan-Meier method will be employed to calculate the PFS and OS. The Cox proportional hazards model will be employed to estimate the HR. These effect evaluation modalities will also be applied to stratify the analysis by BRCA1/2 or Homologous Recombination Deficiency (HRD) status. All patients enrolled in the study who have received at least one dose of tislelizumab will undergo a safety analysis. Generally, descriptive statistics, including measures such as mean, SD, median and range, are used to summarise continuous variables. Frequencies and percentages are presented for categorical variables. The relationship between biomarker expression, clinical efficacy and prognosis will be investigated through the use of both univariate and multivariate Cox analyses, which will allow for a comprehensive assessment of the sensitivity of the biomarkers to various treatments. The data collected from the CRF for each patient will be presented in a systematic manner. In the event of any deviation from the statistical methods described in the protocol, this will be duly noted in the final report. A p value of <0.05 will be considered statistically significant.
Translational research
Tumour samples are obtained from patients during laparoscopic exploration, biopsy and interval cytoreductive surgery (see figure 1 for the details of the sample collection process). These samples will then be subjected to single-cell sequencing, multicolour immunofluorescence and flow cytometry. The immune cells and mesenchymal stromal cells of the tumour microenvironment will be comprehensively evaluated, and the effects of chemotherapy and chemotherapy combined with immunotherapy on the microenvironment will be studied in detail. The findings are expected to facilitate further exploration of the predictive factors of the efficacy of immunotherapy. Additionally, blood and formalin-fixed paraffin-embedded tissue specimens are collected for each participant, which are used for the BRCA1/2 and HRD assay.
Patient and public involvement
It is important to note that patients or the public had no involvement in any aspect of the design, conduct or reporting of this clinical trial.
Ethics and dissemination
This trial was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of the Medical College of Zhejiang University (approval number: 2024-0374). The protocol is registered on ClinicalTrials.gov (identifier: NCT04815408). Written informed consent is obtained from participants prior to enrolment. In the event of harm to a participant resulting from their involvement in the trial, compensation will be provided by the insurance company in accordance with the relevant insurance policy. The study will continue and results will become available when the primary analysis has been completed and the last subject has been followed for 1 year. Presentations at scientific and professional meetings and publication in peer-reviewed journals will disseminate the results of the study.
Discussion
The present study will evaluate the clinical effectiveness and safety profile of NACT in combination with tislelizumab in patients with advanced EOC. Furthermore, we will examine the correlation between the tumour microenvironment and therapeutic response, as well as the dynamic alterations in the intratumoral immune microenvironment.
Ovarian cancer continues to represent a considerable challenge within the field of gynaecological oncology. The disease is characterised by a high rate of recurrence and a poor prognosis, particularly in advanced stages.16 The utilisation of NACT has emerged as a novel approach to reduce tumour burden prior to surgery, thereby enhancing surgical outcomes and potentially increasing OS. Tislelizumab, an anti-PD-1 monoclonal antibody, has demonstrated encouraging efficacy in the treatment of various cancers by enhancing antitumour immunity. The combination of NACT with tislelizumab has the potential to generate a synergistic effect by reducing tumour size through cytotoxic mechanisms and enhancing the immune system’s capacity to recognise and attack residual cancer cells. This combination has the potential to enhance pathological response and OS in patients with advanced ovarian cancer.
It is imperative to evaluate the safety profile of this combination, given the potential for additive toxicities. A substantial body of research has demonstrated that both NACT and ICIs exhibit manageable adverse event profiles when administered as monotherapy.12 17 Nevertheless, the combination of these two therapies may present unique challenges, such as an increased incidence of immune-related AEs. Consequently, the present study will closely monitor AEs in order to determine the feasibility and tolerability of this treatment regimen in the target patient population.
The ovarian cancer microenvironment (TME) is a complex entity that plays a critical role in disease progression and response to treatment. Consequently, it is an important oncological research area. The TME is constituted by a heterogeneous cellular milieu, encompassing immune cells, stromal cells and the extracellular matrix. These elements interact with tumour cells, thereby modulating treatment outcomes.18 It is imperative to understand how the TME influences response to NACT and tislelizumab in order to identify predictive biomarkers and potential therapeutic targets. The present study will investigate the correlation between TME characteristics and therapeutic response by analysing tumour samples before and after treatment. The study will place particular emphasis on the presence, activity and impact of tumour-infiltrating lymphocytes, the expression of immune checkpoint molecules and the density of immunosuppressive cells. This will include regulatory T cells, M2 macrophages and myeloid-derived suppressor cells. The results of these analyses will help identify which aspects of the TME are most predictive of a favourable response to the combination therapy. The potential of immune checkpoint blockade to induce dynamic changes in the intratumoural immune microenvironment is another key area of research. Such changes may result in the conversion of an immunosuppressive environment to one that is more conducive to antitumour immunity.19 20 By monitoring these changes over the course of treatment, we can elucidate the underlying mechanisms of response and resistance to therapy.
In conclusion, this study aims to provide a foundation for the combination of NACT with tislelizumab in advanced ovarian cancer. This will contribute to a deeper understanding of the clinical effects, safety profile and fundamental immunological processes. The findings will contribute to the growing body of evidence in support of the incorporation of immunotherapy into the treatment paradigm for ovarian cancer, thus facilitating the development of more personalised and efficacious therapeutic modalities.
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
We gratefully acknowledge contributions from our research team for help during the study.
References
Footnotes
YZ and JF contributed equally.
Contributors YZ and JF contributed equally to writing the article. JZho and ZZ developed the study concept and protocol. YZ, JF and XZ assisted in further protocol development. JZha supervises the clinical trial and has access to the final trial data set. All authors contributed to the article and approved the submitted version. ZZ acted as the guarantor.
Funding This study was partially supported by the National Natural Science Foundation of China (82203620).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.