Article Text
Abstract
Objectives To investigate the impact of allopurinol use on the risk of first-ever acute coronary syndrome (ACS) event in patients with gout.
Methods Using national and regional register data, we included all patients with a gout diagnosis at primary or specialised care in Western Sweden in the period 2007–2017 (n=18 862; 67% male patients). Patients with a prior history of coronary heart disease (CHD) were excluded. Follow-up started at the first gout diagnosis and ended at the first-ever ACS event, death or study end. The main outcome was the risk of first-ever ACS in: (1) allopurinol users versus non-users, by defining three categories of allopurinol exposure: exposed to 100 mg, >100 mg and no exposure (reference) and (2) allopurinol initiators (within 125 days) versus long-term users (reference). Multivariable logistic regression analysis was used to calculate ORs and 95% CIs.
Results In analysis 1 (n=18 862), 15.3% (n=2892) were exposed to 100 mg, 9.1% (n=1717) to >100 mg and 75.6% (n=14 253) were not exposed. Allopurinol users were older and had more comorbidities compared with non-users. Allopurinol exposure (100 mg and >100 mg) was associated with significantly lower odds of first-ever ACS (OR 0.77; 95% CI 0.63 to 0.94, and OR 0.61; 95% CI 0.47 to 0.81, respectively). In Analysis 2, allopurinol initiators (n=489) had significantly higher odds of first-ever ACS compared with long-term users (n=2916) (OR 1.68; 95% CI 1.03 to 2.75).
Conclusions In patients with gout and without CHD, long-term allopurinol use protects against first-ever ACS compared with non-users. In contrast, allopurinol initiators, possibly having more systemic inflammation, had a higher risk of first-ever ACS compared with long-term users.
- RHEUMATOLOGY
- Cardiovascular Disease
- REGISTRIES
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study used data from large population-based registers, minimising selection bias.
The data used were derived from both primary and secondary healthcare, covering all possible phenotypes of gout.
Gout cases and acute coronary syndrome events were identified using validated definitions, minimising potential misclassification bias.
Although we controlled for multiple confounders, there is still a risk of residual confounding.
Introduction
Background
Cardiovascular disease (CVD) is the leading cause of death globally, with nearly half of these deaths due to ischaemic heart disease.1
Gout is the most common inflammatory arthritis, with a global prevalence ranging from less than 1% to 6.8% and rising incident rates in many countries.2 Gout patients are at increased risk of CVD, with accumulating evidence suggesting that gout is an independent cardiovascular (CV) risk factor.3–9 The mechanistic hypotheses for why gout might independently increase CV risk include hyperuricaemia and systemic inflammation. Although gout has traditionally been considered an intermittent inflammatory disease, several recent studies have reported persistent inflammation even during asymptomatic periods.10 11 Additionally, hyperuricaemia itself might contribute to endothelial dysfunction.12
The first-line treatment for gout is the urate-lowering drug allopurinol,13 14 which has well-established benefits for joint disease.15–18 It is more controversial whether allopurinol also reduces the risk of CV events, possibly by reducing urate levels or by decreasing xanthine oxidase-mediated vascular oxidative stress.19
Some observational studies reported that urate-lowering therapy (ULT) reduces CV risk,20–22 whereas others did not find such benefits.23 24 Small intervention studies have shown benefits of allopurinol on several CV manifestations, including endothelial function,25–27 blood pressure28–30 and carotid intima-media thickness progression,29 while others have not.31–33 A previously published open-label randomised controlled trial (ALL-HEART study) in patients with ischaemic heart disease did not demonstrate improvement in CV outcomes with allopurinol treatment, but gout patients were excluded.34
Systemic inflammation itself may increase the risk for CV events both in the general population35 and in patients with gout.36 A previous study found that the risk of CV events was temporally increased close to gout flares, suggesting that acute inflammation related to a gout flare increases the risk of CVD events for 6 months.36 A surrogate marker for a gout flare may be ULT initiation.37 The risk of CV events in patients initiating allopurinol compared with long-term treatment has not been previously studied.
Objectives
This study aimed to investigate the impact of allopurinol use on the risk of a first-ever acute coronary syndrome (ACS) event in patients with incident gout and no prior history of coronary heart disease (CHD) by comparing: (1) allopurinol users versus non-users (to measure the overall effect of treatment) and (2) allopurinol initiators versus long-term users (with initiators serving as a surrogate for recent flares) at the time of the ACS event.
Methods
Study design and setting
This is a population-based cohort study of patients with incident gout in Western Sweden in the period 2007–2017.
The healthcare system in Sweden is mainly public, tax-funded and independent of the individual’s insurance or financial status. The patients with gout are usually diagnosed and treated in primary healthcare units and only to a lesser extent, in specialised healthcare units. The 10-digit personal identification number, which is unique for each individual and automatically assigned to every Swedish resident, enables linkage of data from different registers.
The manuscript is an honest, accurate and transparent account of the study being reported. No important aspects of the study have been omitted.
Data sources
The Western Swedish Healthcare Register (VEGA) contains information about all healthcare contacts at both primary and secondary healthcare in Western Sweden (approximately 1.7 million inhabitants) from the year 2000 onwards. All diagnoses given by physicians are registered according to the Swedish version of the International Classification of Disease (ICD) codes. Since 1997, the 10th version of ICD codes is used in Sweden.
The Swedish Prescribed Drug Register contains information about all prescribed drugs dispensed by Swedish pharmacies since July 2005, based on the Anatomical Therapeutic Chemical (ATC) Classification System.
The Cause of Death Register contains information about the date and cause of death for all deceased residents since 1961.
Patient and public involvement
This study was based on register data. Patients or the public were not involved in the study design.
Participants
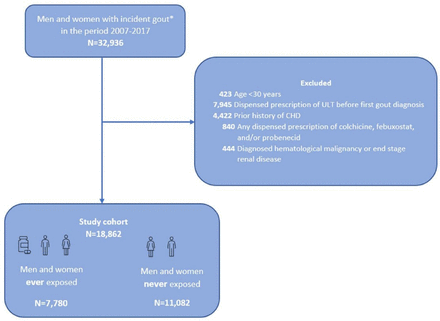
By using data from VEGA, all patients aged ≥30 years in Western Sweden with first gout diagnosis after 1 January 2007, at either primary or secondary healthcare were identified. Cases were regarded as incident, if they did not have any recorded gout diagnosis in the previous 7 years. Cases with prior history of CHD or exposure to allopurinol before first gout diagnosis were excluded (online supplemental table 1). Patients with any dispensed prescription of colchicine, febuxostat or probenecid were excluded. We also excluded patients with a history of haematological malignancy and/or end-stage renal disease to minimise confounding by indication (figure 1).
Supplemental material
Design of study cohort. *Defined as no recorded diagnosis of gout in the previous 7 years. CHD, coronary heart disease; ULT, urate-lowering treatment.
Outcome of interest
The study outcome was the first-ever ACS event, defined as the first reported ICD-coded primary diagnosis of either myocardial infarction (MI) or unstable angina at discharge from an inpatient unit or ACS as primary cause of death without prior non-fatal ACS (online supplemental table 1). By narrowing our focus on ACS and not on other CV outcomes, we aimed to conduct a study which provides robust results regarding the relationship between allopurinol use and acute coronary events. The follow-up began on the first ICD-coded diagnosis of gout and ended on the earliest of the outcomes, emigration, death or the end of study on 31 December 2017.
Analysis 1: users versus non-users
The exposure of interest was the prescription of allopurinol within 125 days before the end of follow-up. The authors considered 100 days of treatment, for which allopurinol prescription in Sweden is usually prescribed, allowing up to 25 days of grace period. They defined three different levels of exposure based on daily dose: 100 mg daily, >100 mg and no exposure (figure 2, I).
Study exposure of interest. (I) Analysis 1: Users versus non-users at the end of follow-up. (A) Exposure to 100 mg. (B) Exposure to >100 mg. (C) No exposure (reference group). (II) Analysis 2: Initiators versus long-term users. (A) Initiators, defined as having dispensed a prescription of allopurinol within 125 days before the end of follow-up and no dispensed prescription during a look-back period of 365 days. (B) Long-term users, defined as having dispensed a prescription of allopurinol within 125 days before the end of follow-up and having continuous allopurinol treatment during a look-back period of 365 days. ACS, acute coronary syndrome.
Analysis 2: initiators versus long-term users
We defined two categories of exposure, allopurinol initiators and long-term users. Both categories were exposed to allopurinol at the end of follow-up, defined as having dispensed a prescription of allopurinol within 125 days before the end of follow-up. The initiators were defined as not having dispensed a prescription of allopurinol during a look-back period of 365 days and the long-term users as having continuous allopurinol treatment during the same time period (figure 2, II). The quantity dispensed and number of days supplied from each filled prescription were used to calculate the proportion of days on which a patient had allopurinol available (proportion of days covered (PDC)). Continuous treatment as a PDC of at least 80% in the given interval was then defined (figure 2, II).
Sensitivity analysis
To delineate the long-term effect in those on chronic allopurinol treatment from that in allopurinol initiators, a sensitivity analysis excluding initiators from those exposed to allopurinol at the time of the ACS event in analysis 1 was also performed.
Covariates and confounders
The authors included comorbidities which could be possible confounders because of either the medical condition itself or its treatment (hypertension, diabetes, hyperlipidaemia, obesity, renal disease, heart failure, cardiomyopathy, psoriasis, chronic obstructive pulmonary disease (COPD), alcoholism, cerebrovascular disease, atherosclerotic disease and cancer). Comorbidities were assessed based on the presence of the respective ICD code during the follow-up period. The diagnoses of diabetes, hyperlipidaemia and obesity were further identified if they had at least one ATC-coded dispensed prescription of anti-diabetic, lipid-lowering or anti-obesity drugs, respectively (online supplemental table 1). Comorbidities were included in the analyses as comorbidity index based on the number of ever diagnosed comorbidities during the follow-up: 0, 1–2, 3–4 or >5.
Medication was defined as a dispensed prescription of anticoagulants, CV drugs or cortisone within 6 months before the end of follow-up. The category of CV drugs included vasodilator drugs, anti-hypertensive drugs, diuretics, beta-blockers, calcium antagonists and renin-angiotensin-aldosterone inhibitors (online supplemental table 1). Exposure to non-steroidal anti-inflammatory drugs (NSAIDs) or Cox-2 inhibitors was not included in the analyses, due to uncertain exposure (unknown amount sold over the counter).
Statistical methods
Continuous variables are presented as mean±SD. Categorical variables are presented as number and percentage. Comparisons between continuous variables were performed with analysis of variance and between categorical variables with the Kruskal-Wallis test.
Unadjusted and adjusted logistic regression analysis were performed to calculate ORs and 95% CIs for the first-ever ACS event. Multivariable logistic regression analysis was performed with adjustments for age, sex, education level, comorbidity index and medication. The unexposed category was used as the reference group in analysis 1, and the long-term users were used as the reference group in analysis 2.
Statistical analyses were performed by using SAS V.9.3 (SAS Institute, Cary, North Carolina, USA).
Results
Participants
After exclusions for age (n=423), ULT treatment before gout diagnosis (n=7945), prior history of CHD (n=4422), treatment with colchicine, febuxostat or probenecid (n=840) and diagnosed haematological malignancy or end-stage renal disease (n=444), a total of 18 862 patients with incident gout were included in this study (67% men) (figure 1). Of these, 41.2% (n=7780) had at least one dispensed prescription of allopurinol and 58.8% (n=11 082) had no dispensed prescription of allopurinol during the total follow-up period (figure 1).
Main results
Analysis 1: users versus non-users
Among the patients with incident gout included in this analysis (n=18 862), 15.3% (n=2892) were exposed to 100 mg allopurinol, 9.1% (n=1717) were exposed to >100 mg allopurinol and 75.6% (n=14 253) were not exposed to allopurinol within 125 days before the end of follow-up (table 1). The mean allopurinol dose in the group exposed to >100 mg was 260 mg in men and 246 mg in women.
Demographic and clinical characteristics of patients with incident gout included in analysis 1
Patients exposed to allopurinol were older and had more comorbidities and medication, as compared with those not exposed. No significant differences were observed between patients exposed to 100 mg and >100 mg allopurinol regarding age, comorbidity index or medication (table 1, online supplemental table 2).
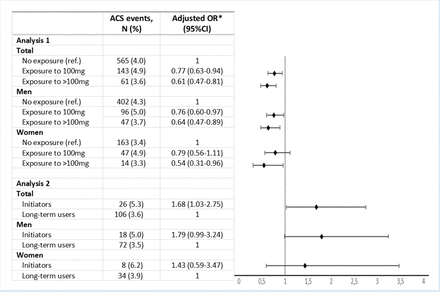
In the adjusted model, patients exposed to allopurinol had significantly lower odds of ACS event, compared with those not exposed (OR 0.77; 95% CI 0.63 to 0.94 for 100 mg and OR 0.61; 95% CI 0.47 to 0.81 for >100 mg). Compared with low dose of allopurinol (100 mg), a higher dose (>100 mg) was associated with lower odds of ACS event, but the difference was not statistically significant (p value for trend, 0.16). In women, exposure to >100 mg was associated with significantly lower odds of ACS event, whereas exposure to 100 mg did not reach statistical significance (figure 3).
Association between allopurinol exposure and acute coronary syndrome (ACS) events, total and by sex. Analysis 1: users versus non-users. Analysis 2: allopurinol initiators versus long-term users. *Adjusted for age, education level, comorbidity index and medication.
Analysis 2: initiators versus long-term users
Overall, 489 initiators and 2916 long-term users were included in this analysis. Initiators were less likely to be treated with anticoagulants and CV drugs, but more likely to be treated with cortisone, compared with the long-term users (table 2). Male initiators were more likely to have renal disease and COPD and less likely to have hypertension compared with male long-term users. Female initiators were more likely to have renal disease, heart failure and COPD compared with female long-term users (online supplemental table 3).
Demographic and clinical characteristics of patients with incident gout included in analysis 2
In the adjusted multivariate logistic regression analyses, initiators had significantly higher odds of ACS events compared with long-term users, after adjustments for age, education level, comorbidity index and medication (figure 3).
Sensitivity analysis
After excluding allopurinol initiators (N= 489), 4120 patients exposed to allopurinol and 14 253 patients not exposed remained. Patients exposed to allopurinol had significantly lower odds of ACS event compared to those not exposed and similar to ORs observed in analysis 1 (OR 0.75; 95% CI 0.61 to 0.93 for those exposed to 100 mg allopurinol, and OR 0.64; 95% CI 0.48 to 0.85 for those exposed to >100 mg), which is probably explained by the low number of allopurinol initiators.
Discussion
In this population-based cohort study, the authors studied the effect of allopurinol use on the risk of first-ever ACS event in patients with incident gout and no prior history of CHD. It was found that allopurinol users had a significantly lower risk of first-ever ACS, whereas the subgroup of recent allopurinol initiators had an increased risk, possibly reflecting recent flares and a higher contemporary level of systemic inflammation. In the dose-dependent analysis, it was found that compared with low dose (100 mg), a higher dose of allopurinol (>100 mg) was associated with lower odds of ACS event, but the difference was not statistically significant.
The first finding provides evidence for a potential benefit of allopurinol in primary CV prevention in patients with gout. Allopurinol reduces urate levels, may decrease systemic inflammation and the generation of oxidative species and may reverse endothelial dysfunction.38 This may explain the association between allopurinol use and the lower risk of ACS found in our study.
ULT initiation is usually started due to active gout with frequent flares and may be associated with an increased risk of gout flares during the initial period after initiation.37 A previously published study showed that gout flares were associated with a transient increase in CV events following the flares, possibly due to neutrophilic inflammation which may cause atherosclerotic plaque instability and rupture.36 This may explain the association between increased risk of ACS events and allopurinol initiation. Allopurinol initiators were more frequently prescribed cortisone compared with long-term users, possibly due to gout flares or as flare prophylaxis. To the best of our knowledge, this study is the first to report an increased risk of ACS events during allopurinol initiation compared with long-term allopurinol use.
Previous observational studies exploring the effect of allopurinol use on CV outcomes in patients with gout have shown conflicting results. Singh et al found that allopurinol use was associated with a lower risk of MI in older patients with gout (≥65 years) and that risk reduction was associated with the duration of treatment.39 Allopurinol use for >6 months to 2 years and over 2 years was associated with lower risk of MI compared with non-use, whereas allopurinol use for <6 months was not. In another study by Singh et al, current allopurinol use, defined as a new filled prescription, was associated with lower risk of MI and stroke in patients with gout and diabetes compared with previous allopurinol use.21
In contrast, the risk of CVD did not differ significantly between the allopurinol and non-allopurinol group in a cohort of gout patients, but 69% of patients in the non-allopurinol group received a uricosuric agent.40 Lin et al showed that allopurinol was not associated with a lower risk of coronary artery disease (CAD) in patients with newly diagnosed gout. However, the dose-stratified analysis showed that the risk of CAD was significantly lower in higher allopurinol doses (>270 defined daily doses (DDDs)) compared with lower doses (0–90 DDDs).41 The doses of allopurinol in our study were relatively low, but these are the doses generally used in clinical practice for gout patients in Sweden, where gout remains suboptimally managed.42 43
The previously published ALL-HEART study showed that allopurinol treatment does not improve CV outcomes in patients with ischaemic heart disease.34 However, the study excluded patients with a history of gout, the mean urate concentration at baseline in the allopurinol group was low (0.34 mmol/L (5.7 mg/dL)) and the discontinuation rate for allopurinol was high. These differences in the selection of treated patients might explain the divergent results between our study and the ALL-HEART study, potentially reflecting different CV outcomes in patients with normouricaemia and those with hyperuricaemia/gout. Moreover, the allopurinol doses used in the ALL-HEART trial were higher (300 mg daily for patients with renal impairment and 600 mg daily for those without) compared with the doses used in our study. The high doses in the ALL HEART trial likely contributed to the early and premature cessation of allopurinol in the majority of participants allocated to allopurinol therapy, which makes the results of this trial inconclusive. In contrast, the current study provides epidemiological support for conducting a new RCT comparing allopurinol with placebo in gout patients, using lower doses tailored to urate response, which are likely better tolerated.
This study had several limitations. First, as with all observational studies, there is a risk of residual confounding. To minimise this bias, the authors controlled for multiple potential confounders. Second, as in other pharmacoepidemiologic studies, it was not certain that allopurinol was taken by the patients. Such misclassification of exposure is, however, likely to be non-differential between exposed groups and in comparison with those unexposed. Poor adherence would probably result in an underestimation of the reduced risk. Third, it was not possible to verify the indication for each allopurinol prescription, but all cases in this study had a diagnosis of gout, and cases with disorders that could indicate allopurinol use for another reason (ie, haematological malignancy or end-stage renal disease) were excluded. Fourth, data on gout severity (ie, tophi, erosions and urate levels) are infrequently reported in the registers and could not be adjusted for in the analyses. However, this may have had only a moderate impact on this study as we included only patients with incident gout. Fifth, in comparisons between allopurinol initiators and long-term users, the study power in the sex-stratified analyses may not have been sufficient to reach statistical significance. Finally, we were not able to adjust for smoking, body mass index, diet, exercise, over-the-counter NSAID use and family history of CVD, because these data are not reported in the registers.
This study had several strengths. By excluding patients with dispensed prescriptions of colchicine, the authors were able to study the isolated effect of allopurinol on the risk of ACS. Furthermore, this study used data from large population-based registers, and the study population is representative of the general population in Sweden.44 45 The data used were derived from both primary and secondary healthcare, covering all possible phenotypes of gout, from mild to severe disease. Gout cases and ACS events identified using validated definitions to minimise potential misclassification bias. Previous validation studies showed high validity for the diagnoses of gout and MI in the Swedish registers.46 47
Conclusions
Among patients with incident gout and no prior history of CHD, allopurinol users had a significantly lower risk of a first-ever ACS event compared with non-users. This finding provides evidence for a potential benefit of allopurinol in primary CV prevention for patients with gout. However, the subgroup of recent allopurinol initiators had a higher risk of a first-ever ACS event compared with long-term users. This finding may reflect a higher frequency of gout flares and systemic inflammation both before and initially after allopurinol initiation. Whether this risk is affected by the use of flare prophylaxis needs to be investigated in further studies.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Ethical approval for the study was granted from the Ethical Review Board of Gothenburg, Sweden, no. 2019-05772. Informed consent from the patients was not needed, as the study was based on register data.
Acknowledgments
Part of these results have been presented as an oral communication at the ACR Convergence in Philadelphia, November 2022, and in the Scandinavian Congress of Rheumatology in Copenhagen, August 2023.
References
Footnotes
Contributors All authors have contributed substantially to the process of completing this study and had full access to the data, specified as follows: PD contributed to the conception and design of the study, managing and interpretation of data, drafting and revising the manuscript. TZS contributed with all statistical analyses, drafting and revising the manuscript. UL and KB contributed to study design, interpretation of data, drafting and revising the manuscript. LJ and MD contributed to the conception and design of the study, as well as interpretation of data and drafting and revising the manuscript. All authors have approved the manuscript. Guarantor: PD.
Funding Funding for the study was received from the following sources, which did not influence either the design of the study, the collection and analysis of the data, or the preparation of the manuscript: Reumatikerförbundet (no. R-995247), the Swedish Heart Lung Foundation (Hjärt- Lungfonden no. 20230630), the Rune och Ulla Amlövs Stiftelse för neurologisk och reumatologisk forskning (no. 2023-420), and Göteborgs Läkarsällskapet (no. 985401). All researchers assigned as authors state their complete independence from the funders regarding this study.
Competing interests All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work. PD has received fees for Advisory Board from Horizon Therapeutics. All other authors have no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years. No other relationships or activities that could appear to have influenced the submitted work.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.