Article Text
Abstract
Introduction The optimal method for removing the endotracheal tube (ETT) during extubation in the intensive care unit (ICU) remains uncertain. Two methods are described for removing the ETT in ICU, namely the ‘Traditional technique’ with continuous aspiration during cuff deflation and ETT removal; and the ‘PEEP’ method, which consists in applying positive end-expiratory pressure (PEEP) before and during cuff deflation and ETT removal. Our hypothesis is that applying PEEP during extubation in the ICU would improve clinical outcome.
Methods and analysis This is a prospective, multicentre, randomised, open-label, controlled, superiority trial, analysed by intention-to-treat, comparing ETT removal with concomitant suction vs application of PEEP before and during ETT removal. In total, 424 patients will be recruited and randomly assigned in a 1:1 ratio to one of two groups, according to the strategy of ETT removal. The primary outcome is the number of days free from any mechanical ventilation within 28 days following extubation. Secondary outcomes include the reintubation rate up to 7 days after ETT removal, the cumulative duration of non-invasive ventilation up to 7 days following extubation, the rate of acute respiratory failure, the rate of acquired pneumonia during the first 7 days following ETT removal, the length of stay in ICU and in hospital and all-cause mortality at 28 days following ETT removal.
Ethics and dissemination The study was approved by the Ethics Committee ‘CPP Ile de France II’. Patients will be included after providing written informed consent. The results will be submitted for publication in peer-reviewed journals, and in national and international congresses.
Trial registration number NCT05147636.
- Adult intensive & critical care
- Respiratory Therapy
- Clinical Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
EXSUPEEP is a large prospective multicentre randomised open-label controlled superiority trial, comparing two techniques for removing endotracheal tube.
EXSUPEEP is designed to show a significant improvement in clinical outcome, assessed by the number of days free from any mechanical ventilation within 28 days after endotracheal tube removal, and a decrease in the incidence of acute respiratory failure or extubation failures.
The EXSUPEEP study aims to be a practical clinical trial with broad clinical eligibility criteria. Study procedures are embedded into routine care and implemented daily by physiotherapists in ‘real-world’ intensive care practice.
The individual study assignments of the patients will not be masked. Given the characteristics of the two strategies under evaluation, a double-blind trial is not possible.
Introduction
Background and rationale
Up to 30% of patients admitted to the intensive care unit (ICU) require mechanical ventilation.1 However, extubation in the ICU is challenging, because of the high incidence of extubation failure.2 3 Among patients at high risk of post-extubation complications, the failure rate is estimated at 15%–20%,3–5 and mortality among patients who undergo reintubation is estimated at 30%–40%.4–8 In one international study of patients who required invasive ventilation for at least two calendar days, only 65% were successfully weaned from invasive ventilation at day 90.5 Therefore, the question arises as to the steps that could be taken to better extubate in the ICU setting. From a technical standpoint, extubating in an ICU can be divided into three steps:9
First, one must assess the patient’s eligibility for weaning from mechanical ventilation and obtain the clinical criteria to enable extubation.10 11
Second, a spontaneous breathing trial (SBT) must be performed to simulate post-extubation conditions.3 12 This trial must be performed for all patients who have undergone at least 24 hours of invasive ventilation. Clinicians choose between a T-piece trial, disconnecting the patient from the ventilator, or a pressure-support ventilation (PSV trial), without disconnecting the patient from the ventilator and using a very low level of PSV.
The third step is the removal of the endotracheal tube (ETT).
While the two first steps have been extensively studied,3 10–13 the optimal method for removing the ETT remains uncertain.
Currently, two extubation methods are described in the literature for removing an ETT.14 15 The traditional method consists in removing the ETT with concomitant endotracheal aspiration. First, a chest physiologist or nurse introduces a suction catheter into the ETT. Then, continuous suction is applied, starting from the deflation of the cuff and until the complete removal of the ETT.
The second method used for ETT removal consists in first aspirating, then applying positive expiratory pressure from cuff deflation to removal of the ETT. Recent studies have shown that applying positive pressure during extubation is safe16 and seems to be non-inferior to traditional extubation methods.16
While most critical care professionals use endotracheal suctioning from cuff deflation to removal of the ETT,14 17 several laboratory studies have shown that suction during extubation does not minimise leakage of oropharyngeal contents, but actually results in greater leakage.18 19 In fact, after deflating the cuff, secretions accumulated in the subglottic space during invasive ventilation could pass into the airways during endotracheal aspiration because of the negative pressure generated by suction. When positive end-expiratory pressure (PEEP) is applied, leakage values seem to be lower, especially for PEEP values of more than 10 cm H2O.19 Furthermore, applying PEEP might produce a beneficial effect on alveolar recruitment.
We aim to conduct a prospective multicentre randomised open-label controlled superiority trial, analysed by intention-to-treat, comparing two techniques for ETT removal in the ICU, namely extubation with removal of the ETT while aspirating, versus application of PEEP during cuff deflation and extubation. Our hypothesis is that applying PEEP during cuff deflation and extubation may improve clinical outcomes.
Primary objective
The primary aim is to evaluate whether applying PEEP during cuff deflation and extubation will increase the number of days free from any mechanical ventilation compared with extubation removing the ETT with concomitant endotracheal aspiration.
Secondary objectives
Secondary objectives are to demonstrate that applying PEEP during cuff deflation and extubation makes it possible to:
Decrease the incidence of extubation failures.
Reduce the use of non-invasive ventilation (NIV) and/or high flow oxygen therapy (HFO).
Reduce the incidence of post-extubation pneumonia and/or atelectasis.
Decrease the incidence of acute respiratory failure.
Reduce the length of stay in intensive care and/or in hospital.
Decrease mortality at 28 days.
Trial design
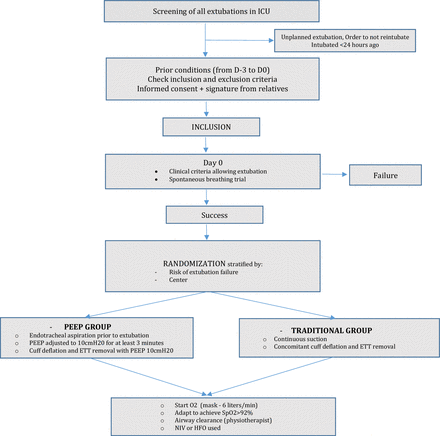
EXSUPEEP is a randomised, controlled open-label trial comparing two strategies of ETT removal, namely the traditional method, consisting of ETT removal with concomitant endotracheal suction versus the experimental method, consisting of application of PEEP from before cuff deflation up to complete removal of the ETT. Patients will be randomised in a 1:1 ratio to one of the two groups according to the flow chart detailed in figure 1.
Flow chart of the patients and study design. ETT, endotracheal tube; HFO, high flow oxygen; ICU, intensive care unit; NIV, non-invasive ventilation; PEEP, positive end-expiratory pressure.
The EXSUPEEP study aims to be a practical clinical trial with broad clinical eligibility criteria. Study procedures are embedded into routine care and implemented daily by physiotherapists.
Methods: participants, intervention and outcomes
Study setting
The EXSUPEEP Study will be conducted between March 2023 and January 2025 in 11 ICUs in France.
Eligibility criteria
Inclusion criteria
First, the decision to extubate must be made by the treating clinicians after having validated that the clinical criteria for weaning are met, namely: according to the international conference consensus on weaning,20 patients will be considered as ready for an initial SBT as soon as they meet all of the following criteria:
Respiratory rate ≤35 breaths per minute.
Adequate oxygenation defined as pulse oximetry (SpO2 ≥90%) with a fraction of inspired oxygen (FiO2) ≤0.4 or PaO2/FiO2 ≥150 mm Hg with PEEP ≤8 cm H2O.
Haemodynamic stability with no need for vasopressors (or doses ≤0.3 µg/kg/min).
Adequate cough.
Patient awake with a Richmond Agitation-Sedation Scale between +1 and −2.21 22
Patients meeting any of the following criteria will be included in the EXSUPEEP trial:
ICU hospitalisation.
Oro-tracheal intubation.
Mechanical ventilation for more than 24 hours.
First extubation procedure during the stay in the including unit.
Consent collected from a relative of the patient.
Once it has been verified that the patient meets all the eligibility criteria listed above, the patient may be extubated after a successful SBT and after obtaining consent from the patient or family.
Exclusion criteria
Patients meeting any of the following criteria will be excluded from the EXSUPEEP trial:
Patients receiving ventilation via tracheostomy.
Patients with underlying chronic neuromuscular disease.
Patients with severe head injury.
Patients with a decision to withhold and/or withdraw life support.
Patients not affiliated to or beneficiary of any social security scheme.
Person benefiting from enhanced protection, namely minors, pregnant or nursing women, persons deprived of their liberty by a judicial or administrative decision, persons residing in a healthcare or social establishment, adults under legal protection (safeguard of justice, guardianship or curatorship).
Inclusion in another research project that interferes with the outcomes of the present study.
Weanability criteria not met within 72 hours following the signing of consent by the relatives.
Unsuccessful weaning test within 72 hours following the signing of consent by the relatives.
Patients with personal NIV or continuous positive airway pressure at home will be included if pressure support (PS) and/or PEEP used in the ICU are different from the usual settings at home. Regarding infection by SARS-CoV-2 or the use of ETTs with subglottic suction, they do not constitute an exclusion criterion.
Study intervention
First, patients must be reconnected to mechanical ventilation for at least 1 hour after a successful SBT.23 Although the minimal duration of mechanical ventilation is 24 hours, we expect included patients to receive mechanical duration for more than 72 hours, on average.
Patients eligible for inclusion will be included and then randomised at the time of the decision for planned extubation. Patients will be assigned to one of the two groups as follows: patients assigned to the control group will have their ETT removed with concomitant aspiration. Patients assigned to the intervention group will first undergo aspiration in the endotracheal tube and, then, will be exposed to PEEP for at least 3 min before and during cuff deflation until removal of the ETT.
In case of the use of endotracheal tubes with subglottic suction, the subglottic suction line will no longer be used after randomisation.
In both groups, prior to beginning extubation, patients are straightened and have their chest raised up to more than 60°. Physiotherapists conduct a respiratory physiotherapy session to declutter the patient. Secretions in the mouth will be aspirated. Kits for oxygenation and suction are prepared and tested before extubation.
The exact composition of the respiratory physiotherapy session is at the discretion of each practitioner, according to local practice at each ICU participating in the EXSUPEEP study. A chest physiologist must be present during extubation for all patients included in the trial.
Control group: concomitant aspiration
First, immediately prior to planned extubation, a nurse and chest physiologist must remove the intubation tube fixation system. After introducing a flexible endotracheal catheter into the ETT, they will apply continuous suction from the deflating of the cuff to the removal of the ETT.
Intervention group: PEEP before and during removal of the ETT
First, immediately prior to planned extubation, a nurse and chest physiologist must introduce a flexible endotracheal catheter to suction airway secretions. Then, the ICU ventilator must be adjusted to PSV with PEEP of 10 cm H2O, applied for at least 3 min. PS level is at the discretion of the practitioners, but investigators will be encouraged to target a tidal volume of 6–8 mL/kg. FIO2 will be adjusted to obtain adequate oxygenation (SpO2 >92%). After at least 3 min with PEEP at 10 cm H2O, the endotracheal tube fixation system will be removed. Then, the chest physiologist deflates the cuff and removes the ETT.
In both groups, immediately after the planned removal of the ETT, oxygen therapy is set at 6 liters and rapidly adjusted to obtain SpO2 >90%. The chest physiologist then resumes decluttering of the patient’s respiratory system.
Initiation of NIV or HFO is allowed, but no earlier than 5 min after removal of the ETT, to allow time to declutter. The decision to introduce NIV or HFO is at the clinician’s discretion, according to the official American Thoracic Society (ATS)/American College of Chest Physicians (ACCP) Clinical Practice Guidelines,12 patient characteristics and local practice in each centre.
Outcomes
Primary outcome
The primary endpoint is the number of mechanical ventilation-free days (invasive and non-invasive) within 28 days following the first extubation procedure. Patients who either died or were never extubated and are still under mechanical ventilation at Day 27 will be counted as having zero ventilator-free days from D0 to D27.24
Secondary outcomes
The secondary endpoints are as follows:
The re-intubation rate (%) within 7 days following the removal of the ETT.
The cumulative duration of NIV and HFO, expressed in hours, up to 7 days following extubation.
The proportion of patients with hospital-acquired pneumonia within 7 days following ETT removal (see definition in box 1).
The proportion of patients with pneumonia and/or atelectasis on radiological assessment (%), within 72 hours and within 7 days following the removal of the ETT.
The rate of acute respiratory failure (see definition in box 1) during the first 7 days following ETT removal (%).
Length of stay in the ICU and in hospital, in days.
All-cause mortality within 28 days following ETT removal (from D0 to D27).
Definitions used in outcomes
High risk of extubation failure:
Patients under mechanical ventilation for more than 7 days.
Obesity (Body Mass Index (BMI)>30 kg/m2).
Patients aged older than 65 years.
Patients with underlying chronic lung diseases, including chronic obstructive pulmonary disease, restrictive pulmonary diseases or obesity hypoventilation syndrome.
Patients with underlying chronic cardiac diseases, including atrial fibrillation, documented ischaemic heart disease, history of cardiogenic pulmonary oedema or impaired left ventricular function, defined as left ventricular ejection fraction <45%.
These criteria for high risk of extubation failure must be documented or highly suspected by the physician in several situations: clinical evaluation by the physician (pathologic spontaneous breathing before intubation, rib cage deformation, typical morphotype…); patients intubated for acute hypercapnic respiratory failure; emphysema on chest ray or CT scan.
Criteria for hospital acquired pneumonia is defined as the presence of at least three of the following criteria:
Gas exchange disorders with PaO2/FIO2 <200 mm Hg (with FIO2 in percent=21+(0.3×number of litres of O2)).
Fever ≥38.5°C.
Purulent secretions.
Leukocytosis ≥10 000 by mm3.
Radiological infiltration(s).
Criteria for postextubation respiratory acute failure is defined as the presence of at least two of the following criteria:
Respiratory rate above 30 breaths per minute.
Significant oxygen requirements (O2 flow more than 6 liters by minute, HFO with FIO2 more than 60%).
Clinical signs suggesting respiratory distress with increased accessory muscle activity.
Tachycardia of more than 120 beats per minute.
Deep hypotension, mottling, skin recoloring time more than 5 s.
Agitation, confusion, drowsiness, unconsciousness.
Criteria for postextubation respiratory acute failure needing immediately re-intubation is defined as the presence of any one or more of the following criteria:
Cardiac arrest.
Respiratory exhaustion.
Neurologic failure: coma or uncontrollable agitation.
Refractory haemodynamic failure.
NIV failure: serious intolerance; major complications linked to NIV making it impossible to follow the treatment; deep respiratory acidosis despite NIV defined as pH <7.25 units and increase of PaCO2.
Refractory respiratory failure defined as a need for FIO2 more than 60% to maintain SpO2 level at least 92% or a PaO2/FIO2 ratio <100 mm Hg.
Persistent laryngeal oedema despite medical treatment, endangering the patient.
Sample size
In designing the EXSUPEEP trial, we aimed to identify an absolute increase of 2 days in the average number of ventilator-free days up from D0 to D27, between the two ETT removal techniques (traditional vs interventional method).
Based on the results of studies carried out in similar populations,3 4 25 26 we estimate the number of ventilator-free days from D0 to D27 at 23±7 days.
We therefore hypothesise that the application of PEEP during removal of the ETT will increase the average number of ventilator-free days from D0 to D27, by 2 days (ie, from 23 to 25 days), corresponding to an increase of 9.2%. Estimating the SD of the number of ventilator-free days in this population to be 7 days, with 80% power and an alpha risk of 5%, it is necessary to include 193 patients per group. Allowing a margin of 10% to account for patients lost to follow-up or who withdraw consent, we intend to include 212 patients in each group, that is, 424 patients in total.
Recruitment
In 2021, the ExSUPEEP project received grants from the national ministry of health (PHRIP N°20-0256). The study protocol was approved by the ethics committee ‘Comité de Protection des Personnes Ile de France II’ under the registration number 2022-A00334-39 on 4 November 2022. Patient enrolment started on 22 March 2023 (first patient in). As of 20 May 2024, a total of 257 patients have been included. The anticipated end of inclusions is December 2024.
Randomisation, intervention, data collection and analysis
Randomisation
Computer-generated randomisation will be performed with stratification by centre and by risk factors for extubation criteria (see box 1 for definition).
After randomisation, the extubation strategy assigned to the patient must be initiated within 3 hours.
Intervention
After obtaining consent from the relatives of the patient, all inclusion/exclusion criteria will be verified by the investigator before randomisation. Randomisation will be carried out by connecting to the electronic case report form (e-CRF) website https://ecrf-hcl.ennov.com/EnnovClinical/
Data collection
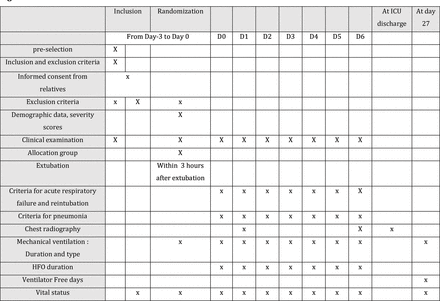
In each centre participating, a trained investigator or research assistant will collect all data on the electronic case report form (e-CRF). Patient follow-up and the data collected at each timepoint are detailed in the study flow chart (figure 2). Data will be stored in a secure online database. The following variables will be recorded for all participants.
Characteristics
Age, gender, Simplified Acute Physiology Score II score, activities of daily living, Sequential Organ Failure Assessment score during the first 24 hours in the ICU.
Flow chart of study showing variables collected at each timepoint. HFO, high flow oxygen; ICU, intensive care unit.
Day of hospital admission, day of admission in the ICU, day of intubation.
Baseline
Clinical parameters just before extubation, namely: Richmond Agitation Sedation Scale, heart rate, systolic, diastolic and mean arterial pressure, respiratory rate, oxygen saturation and oxygen inspiratory fraction (FIO2), and whether or not the patient was connected to the ventilator for at least 1 hour after the weaning trial and before extubation.
After ETT removal: did the patient receive the technique of the group to which they were randomised (yes or no).
At 5 and 60 min after extubation: same parameters as before extubation plus: oxygen flow rate; broncho-pulmonary obstruction (presence or absence); functional cough (yes/no), NIV/HFO during the first hours after extubation.
From Day 0 to Day 6: Each day, investigators must record the patient’s vital status. For each day, it will be noted whether the patient is free from any type of mechanical ventilation (invasive or non-invasive). Similarly, it will be noted every day whether patients have respiratory failure, whether they have been re-intubated or whether NIV/HFO has been started. For each day, signs suggesting a diagnosis of acquired pneumonia should be sought. For NIV and HFO, exposure duration (in hours) per day will be recorded. Chest radiography will systematically be performed on Day 2 and Day 6, and radiologic assessment of pneumonia and/or atelectasis will be performed.
From Day 0 to Day 27: Investigators will record the length of stay in the ICU and in hospital. For each day, dead or alive status will be recorded, regardless of the cause of death if death occurs. Additionally, in order to collect the number of ventilator-free days, exposure to invasive or non-invasive mechanical ventilation will be recorded every day from D0 to D27. Finally, investigators must collect all adverse events occurring during the study period.
Statistical methods
Statistical analyses will be performed by the biostatistics-bioinformatics department of the Hospice Civil de Lyon, France. It will take into account any modification of the protocol or any unexpected event occurring during the study and that has an impact on the analyses according to the predefined statistical analysis plan drafted. The statistical software used will be R V.4.03 (or later versions).
Descriptive analysis of patient groups at baseline
The EXSUPEEP trial is a superiority study with a significance threshold of 0.05. All analyses will be performed on an intention-to-treat basis. Deviations from the protocol will be analysed case-by-case. If patients are wrongly included or do not receive the assigned strategy, deviations from the protocol will be described at the end of the trial and a per-protocol analysis will be considered.
To describe the population in both groups at baseline, quantitative characteristics will be summarised using the mean±SD, if normally distributed, or otherwise as median and range (minimum, maximum). Qualitative characteristics will be described as number and percentage in each category. Characteristics at inclusion will be compared between the two groups.
Missing data management
All necessary means will be implemented to recover missing data, especially for the assessment of the main endpoint. No imputation will be conducted to replace data that is missing during patient follow-up. In the event of missing or invalid data on the judgement criteria, the scientific committee will examine each situation and decide whether the data should be taken into account in the analysis or not.
Primary endpoint definition and calculation of ventilator-free days
The calculation of ventilator-free days counts 1 day free if no support ventilation is used at all during the 24 hours of the calendar day. Each day is considered as a calendar day, apart from D0 which begins at the time of randomisation and ends at 23:59. The day of extubation is identified as D0 and always counted among the days with ventilation. Thus, no patient will have 28 ventilator-free days (the maximum possible will be 27). Deceased patients will be deemed to have 0 ventilator-free days, because of the heavy penalty for deaths in the calculation rule, in line with recent literature.24
Analyses of the primary endpoint
The effect of the intervention on the primary endpoint will be estimated using linear regression adjusted for the two stratification criteria (centre and risk factors for extubation failure). The ventilator-free days probability distribution is typically a mixed distribution (with a peak at zero for deceased patients and then a continuous distribution) requiring a Mann-Whitney-Wilcoxon test for non-parametric continuous values. The effect of the treatment will be quantified by a difference in the primary endpoint between the two groups with a 95% CI. No interim analysis of the primary endpoint is planned.
Analyses of the secondary endpoints
Secondary endpoints using continuous quantitative criteria will be analysed using a linear regression model adjusted for the two stratification criteria. For secondary endpoints using binary criteria, a logistic regression model (also adjusted for stratification criteria) will be used. For survival data, a Cox model adjusted for stratification criteria will be used.
The reintubation rate within 7 days after extubation (from Day 0 to Day 6), the incidence of pneumonia or atelectasis within 72 hours (from Day 0 to Day 2) and within 7 days following the removal of the ETT (from Day 0 to Day 6) will be expressed as means and percentages in the two groups and compared using the χ2 test. The analysis will subsequently be completed by a multivariate logistic regression after testing for interactions. NIV used during the first 7 days, expressed in days and in hours, the length of stay in the ICU and the length of stay in hospital will be compared between treatment groups using Student’s t-test.
The incidence of severe acute respiratory failure and pneumonia within the first 7 days will be expressed as number, and percentage per day, from D0 to D6 and will also be compared between the two groups using Student’s t-test. For each value, Kaplan-Meier curves will be plotted to assess the time from enrolment to the emergence of severe acute respiratory failure or pneumonia.
Mortality at 28 days will be compared between groups, and Kaplan-Meier curves will be plotted to assess the time from enrolment to death. Groups will be compared using the log-rank test.
Predetermined subgroup analysis
To determine the effect of the intervention (PEEP before and during cuff deflation and ETT removal) on patients at high risk of extubation failure,27 28 randomisation will be stratified according to the existence of any underlying cardiac or lung disease, duration of mechanical ventilation (more or less than 7 days before extubation), body mass index (BMI, > or <30 kg/m²) and age (older or younger than 65 years). A subgroup analysis will be done including these criteria as well as severity and health scores for patients at inclusion. Prior to adjustment, an interaction test will be performed to detect heterogeneity of the intervention effect between patients with or without high risk of extubation failure. If the interaction test is significant, results will be presented for the two subgroups separately.
Data monitoring
In each centre participating in the EXSUPEEP study, investigators must screen all extubated patients in the ICU. To check adherence to the protocol and to verify the accuracy of the data recorded, regular monitoring will be planned. Monitoring will be performed by the research staff of the Hospice Civil de Lyon. A Clinical Research Associate (CRA), mandated by the sponsor, will ensure the proper completion of the study, the collection of the data generated in writing, and accurate documentation and recording. Monitoring CRAs will also ensure compliance with Good Clinical Practices as well as the legislative and regulatory provisions in force.
Ethics and dissemination
The EXSUPEEP study is sponsored by the General Hospital of Bourg en Bresse. The study protocol was approved by the ethics committee ‘Comité de Protection des Personnes Ile de France II’ under the registration number 2022-A00334-39 on 4 November 2022.
Consent to participate
In the EXSUPEEP study, according to the decision of the ethics committee ‘CPP Ile de France II’, ventilated patients are unable to provide consent. Patients cannot be included without written consent signed by a next of kin. To continue the trial after recovery, each patient included will be approached to provide continued consent to pursue the trial. Copies of the consent forms are provided in the online supplemental files 1 and 2.
Supplemental material
Supplemental material
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this study.
Access to data
All investigators will have access to the final data set and will be responsible for the quality of data collected. The Hospice Civil de Lyon will provide all data monitoring services, ensuring compliance with the statutory and regulatory provisions in place according to French legislation.
Dissemination policy
Findings will be published in peer-reviewed journals and presented at national and international congresses.
Discussion
Two laboratory studies18 19 have suggested that suction in the ETT during cuff deflation and ETT removal produces greater leakage and increases the risk of aspiration of oropharyngeal secretions into the airways. Application of pressure support ventilation from 5 to 15 cm H2O with PEEP of 5–10 cm H2O reduces the leak volume, with a statistically significant difference.19 In March 2019, Andreu16 showed that applying PEEP of 10 cm H2O before and during cuff deflation and ETT removal was non-inferior to the traditional technique (concomitant aspiration during cuff deflation and ETT removal) in terms of safety, because it did not result in a higher incidence of overall major or minor complications, pneumonia or extubation failure and reintubation rates. Recently, a randomised controlled trial29 that included 725 patients failed to prove that applying PEEP before and during ETT removal could reduce the incidence of major post-extubation complications, despite a trend favouring the PEEP extubation technique. Another study, published in 2023,30 showed that applying 15 cm H2O pressure support with PEEP of 10 cm H2O during ETT removal was associated with persistently better lung aeration and fewer complications compared with the traditional extubation in the 24-hour post-extubation period, but without any effect on pneumonia, ICU-free days or mortality.
However, no study to date has demonstrated the superiority of using positive pressure extubation. One previous study by Andreu et al compared two extubation techniques in critically ill adults, but the primary endpoint did not include either survival or the total duration of mechanical ventilation, and only examined complications occurring immediately post-extubation.29 Therefore, the findings precluded any conclusion as to which technique should be preferred.
The EXSUPEEP study aims to demonstrate that steps taken to prevent leakage of secretions during cuff deflation and ETT removal may increase the number of days free of any mechanical ventilation, compared with an extubation procedure with removal of the ETT under concomitant endotracheal aspiration.
The EXSUPEEP study is a practical clinical trial with broad clinical eligibility criteria. The inclusion criteria are commonplace in the ICU. Study procedures are embedded into routine care and are executed by physiotherapists on a daily basis. NIV or HFO are used according to routine clinical practices in participating centres and in accordance with the French31 and American Thoracic Society12 guidelines. Among extubated patients in the ICU, prophylactic NIV reduces the risk of reintubation within 72 hours, especially for patients with chronic obstructive pulmonary disease.32 While prophylactic HFO is also known to reduce extubation failure after planned extubation in the ICU compared with standard oxygen,33 it has recently been shown that HFO associated with NIV after planned extubation decreased the rate of reintubation for patients at high risk of extubation failure, as compared with HFO alone.34 For the EXSUPEEP trial, we did not lay down guidelines to change practices in terms of NIV and/or HFO. Clinicians participating in the study must offer the best possible treatment for the patient at all times, in accordance with their usual practice. Indeed, the EXSUPEEP trial aims to reflect ‘real-world’ practices in French ICUs.
EXSUPEEP is a prospective multicentre comparative study of two proven techniques, and thus, it will not expose included patients to any additional risk. A recent study has already demonstrated the non-inferiority of applying PEEP during ETT removal,16 with no excess of post-extubation complications. The design of a prospective, randomised, controlled superiority study focusing on efficacy is now justified, and will make it possible to define the most beneficial technique and harmonise practices.
The primary endpoint chosen for this study is the number of ventilation-free days, defined as the number of days free from any form of mechanical ventilation (invasive or non-invasive) within 28 days following ETT removal. Ventilation-free days has been used as an endpoint in several randomised trials focusing on mechanical ventilation in the ICU, especially about extubation3 11 25 34 35 with populations resembling that of EXSUPEEP. We believe that the number of ventilation-free days is particularly suited to our study since it takes into account both extubation failures requiring reintubation, and the use of NIV to prevent extubation failure and death. However, we decided to modify the usual definition of ventilation-free days by including both invasive and non-invasive ventilation. By combining re-intubations, use of NIV and by strongly penalising deaths (by counting zero ventilation-free days if occurs death within 28 days of extubation), we make it possible to obtain an accurate picture real-life ICU practices in terms of mechanical ventilation weaning, focusing on the morbi-mortality associated with extubation failure.
We chose to apply a PEEP level of 10 cmH2O. This PEEP level directly follows laboratory studies,17 19 which showed that PEEP of 10 cm H2O can counterbalance the aspiration gradient, which propels respiratory secretions to the bottom of the respiratory tract during endotracheal suction. This PEEP level also allows alveolar recruitment.
Similarly, in several clinical trials about ETT removal in ICU,29 30 the pressure support level was 15 cm H2O. In the EXSUPEEP study, the pressure support level is modulated to obtain exhaled tidal volumes between 6 and 8 mL/kg of ideal weight relative to the patient’s height. We wish to avoid any volotrauma through exaggerated inspiratory support (Patient Self Inflicted Lung Injury).
We chose to expose patients to PEEP for at least 3 min, based on work conducted on pre-oxygenation before intubation.36 PSV before intubation for at least 3–5 min seems to be sufficient to avoid desaturation and enable adequate alveolar recruitment.
Finally, in our study, patients must be ventilated at least 24 hours to be included in accordance with previous studies focused on extubation in the ICU.
In conclusion, the EXSUPEEP trial is an investigator-initiated randomised controlled trial powered to assess the hypothesis that applying PEEP of 10 cm H2O during cuff deflation and ETT removal may increase the number of days free of any type of mechanical ventilation, in comparison to continuous suction during cuff deflation and ETT removal. This study is a practical clinical trial, designed by physiotherapists. It is underpinned by the hypothesis that an uncomplicated intervention during cuff deflation and ETT removal could protect against pulmonary secretion inhalation, atelectasis or pneumonia after extubation.
By proving with EXSUPEEP that applying PEEP during extubation can increase the number of days free from any ventilation, we hope to reduce extubation failure rates and durably change extubation practices in the ICU setting.
Ethics statements
Patient consent for publication
References
Footnotes
X @Quenot
Contributors NSe is the guarantor. NSe contributed to the study conception and design. NSe, HK, AR, JAP, J-PQ, MS, AL, CD-V, MMe, NT, JB, AB-C, NSt, LM, FS, MMa, GT, PP, AWT, SH, GP, ED, MR, PB, YP and JCB contributed to the data collection and investigation. NSe and J-PQ contributed to the statistical analysis and interpretation. NSe contributed to the writing of first draft. HK, AR, JAP, J-PQ, MS, AL CD-V, MMe, NT, JB, AB-C, NSt, LM, FS, MMa, GT, PP, AWT, SH, GP, ED, MR, PB, YP and JCB contributed to the critical revision for important intellectual content. NSe, HK, AR, JAP, J-PQ, MS, AL, CD-V, MMe, NT, JB, AB-C, NSt, LM FS, MMa, GT, PP, AWT, SH, GP, ED, MR, PB, YP and JCB contributed to the final approval for submission.
Funding This study is funded by the French ministry of health via a grant obtained through the national hospital research programme in June 2021 (Programme Hospitalier de Recherche Infirmier et paramedical, N° 20–0256).
Competing interests The EXSUPEEP team and all investigators do not have any conflicts of interest to declare in connection with the study.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.